Question: Develop expressions for the specific enthalpy and entropy changes [h(v2,T)h(v1,T)],[s(v2,T)s(v1,T)], using the Redlich-Kwong equation of state. Using these expressions, determine the changes in specific enthalpy,
![Develop expressions for the specific enthalpy and entropy changes [h(v2,T)h(v1,T)],[s(v2,T)s(v1,T)], using](https://dsd5zvtm8ll6.cloudfront.net/si.experts.images/questions/2024/09/66f8550440e95_72366f85503dcaca.jpg)
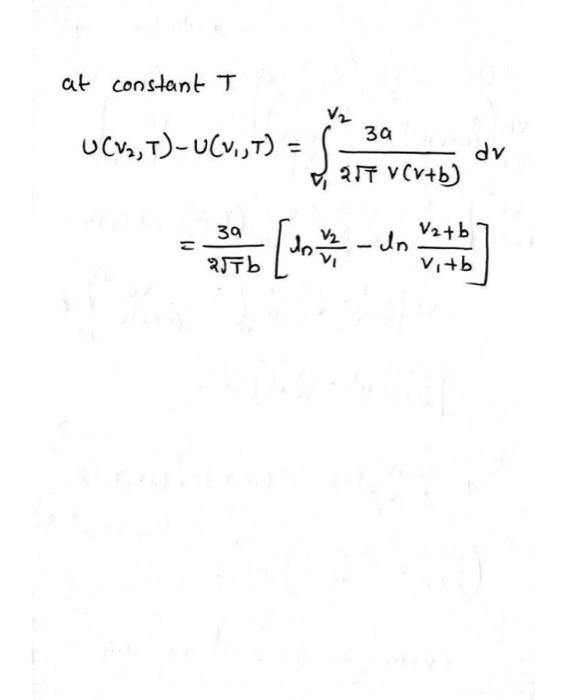
Develop expressions for the specific enthalpy and entropy changes [h(v2,T)h(v1,T)],[s(v2,T)s(v1,T)], using the Redlich-Kwong equation of state. Using these expressions, determine the changes in specific enthalpy, in kJ/kmol, and entropy, in kJ/kmol K, for ethylene between 500K,1 bar and 500K, 150 bar. S= kJ/kmolK h= kJ/kmol atconstantTU(v2,T)U(v1,T)=v1v22Tv(v+b)3adv=2Tb3a[lnv1v2lnv1+bv2+b]
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


