Question: Did I do this right? I am looking for total moles of CO2 lost by P4. Question 4 ( 2 points) You are working to
Did I do this right? I am looking for total moles of CO2 lost by P4.
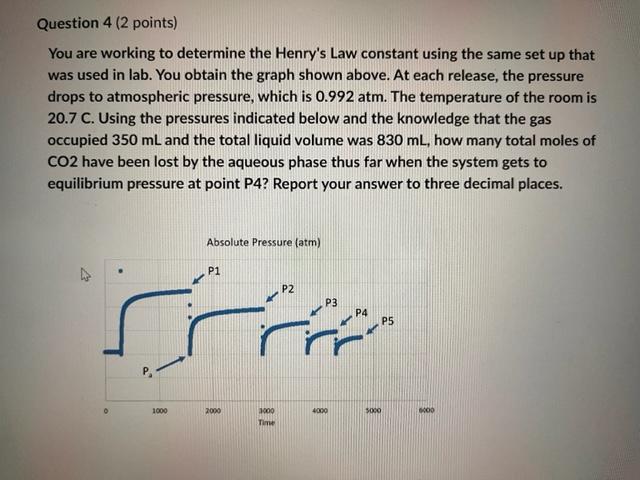
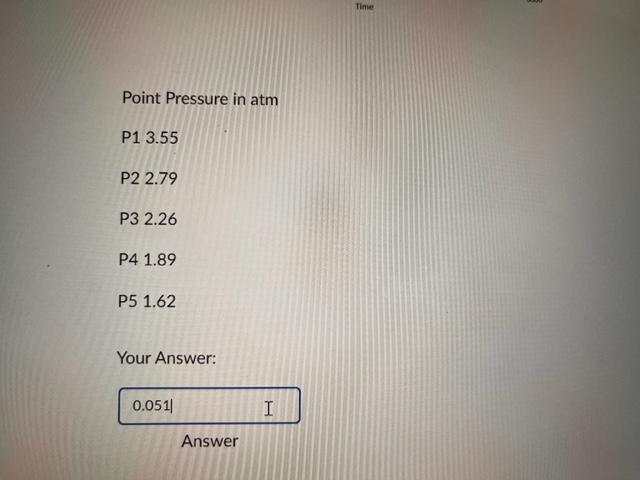
Question 4 ( 2 points) You are working to determine the Henry's Law constant using the same set up that was used in lab. You obtain the graph shown above. At each release, the pressure drops to atmospheric pressure, which is 0.992atm. The temperature of the room is 20.7C. Using the pressures indicated below and the knowledge that the gas occupied 350mL and the total liquid volume was 830mL, how many total moles of CO2 have been lost by the aqueous phase thus far when the system gets to equilibrium pressure at point P4? Report your answer to three decimal places. Absolute Pressure (atm) Point Pressure in atm P13.55 P22.79 P3 2.26 P4 1.89 P5 1.62 Your Answer: 0.051
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


