Question: Find the mass of solid copper(I) chloride that will form when 17.2 mL of 5.15 mol/L copper(I) nitrate is mixed with 21.5 mL of

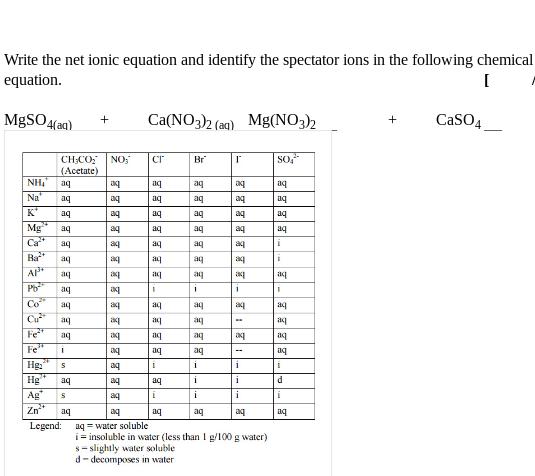
Find the mass of solid copper(I) chloride that will form when 17.2 mL of 5.15 mol/L copper(I) nitrate is mixed with 21.5 mL of 4.81 mol/L lithium chloride. [ /4T] Write the net ionic equation and identify the spectator ions in the following chemical equation. [ MgSO4(aq) 'HN CHCO (Acetate) aq + Ca(NO3)2(aq) Mg(NO3)2 + CaSO4 NO; Cr Br Na aq be be aq be aq aq be FOS be be K aq aq aq aq Mg aq aq aq aq Ca aq be aq aq aq 1 be be aq aq aq Ba aq aq aq aq aq A be aq aq be aq aq P aq be ' 1 i Co aq aq aq aq Cu aq aq aq be Fe aq Fe Hg: Hg Ag Zn I be be aq 22 be aq aq be be be aq aq aq S aq I 1 i ! aq aq aq i i d S aq be be i i i i aq be be Legend: aqwater soluble i insoluble in water (less than 1 g/100 g water) s-slightly water soluble d-decomposes in water
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


