Question: discuss close packing in solid state Osmolarity and Tonicity Practice Problems. (50 points) For a normal person, highlight your answers below to show how body
discuss close packing in solid state 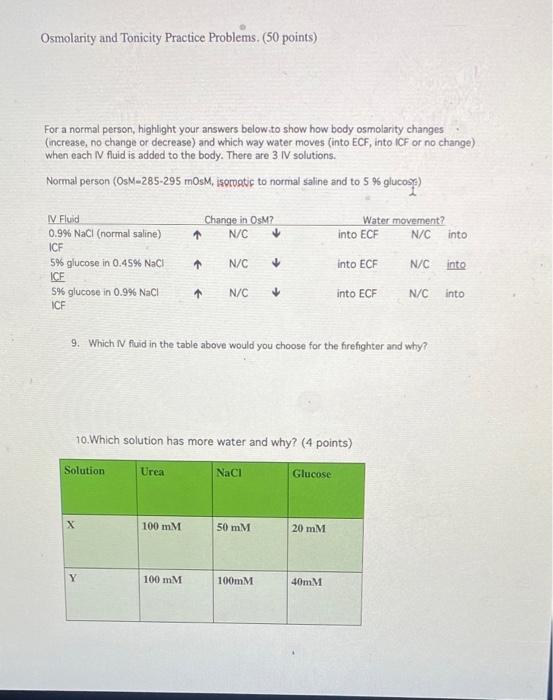
Osmolarity and Tonicity Practice Problems. (50 points) For a normal person, highlight your answers below to show how body osmolarity changes (increase, no change or decrease) and which way water moves into ECF, into iCF or no change) when each IV fluid is added to the body. There are 3. IV solutions. Normal person (OsM-285-295 mOsM, isoratif to normal saline and to 5 % glucose) Change in OsM? N/C Water movement? into ECF N/C into M Fluid 0.9% NaCl (normal saline) ICF 5% glucose in 0.45% NaCH ICF S% glucose in 0.9% NaCl ICF + N/C into ECF N/C into N/C into ECF N/C into 9. Which IV fluid in the table above would you choose for the firefighter and why? 10. Which solution has more water and why? (4 points) Solution Urea Naci Glucose X 100 mm 50 mM 20 mM Y 100 mm 100mM 40mM
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


