Question: DO NOT USE state functions; show all your work and justify every step of your calculations. P T Pz Air at 100 kPa and
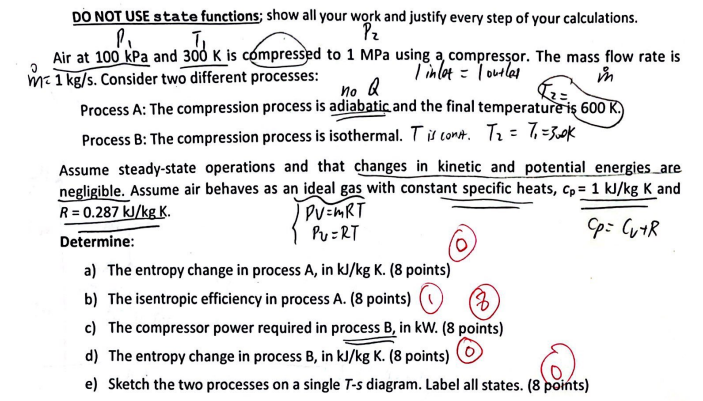
DO NOT USE state functions; show all your work and justify every step of your calculations. P T Pz Air at 100 kPa and 300 K is compressed to 1 MPa using a compressor. The mass flow rate is | inlet = | outlas m m 1 kg/s. Consider two different processes: no Q Process A: The compression process is adiabatic and the final temperature is 600 K.) Process B: The compression process is isothermal. T is cont. T = 7 = 300k Assume steady-state operations and that changes in kinetic and potential energies_are negligible. Assume air behaves as an ideal gas with constant specific heats, Cp = 1 kJ/kg K and R = 0.287 kJ/kg K. Determine: PV=MRT PU=RT a) The entropy change in process A, in kJ/kg K. (8 points) b) The isentropic efficiency in process A. (8 points) c) The compressor power required in process B, in kW. (8 points) d) The entropy change in process B, in kJ/kg K. (8 points) e) Sketch the two processes on a single T-s diagram. Label all states. (8 points) Cp: Cu+R
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


