Question: do we include AlCl3 when solving?? Could you also help solve this problem. 1 1.00 g of benzene, 1.30 g of acetyl chloride, 0.3 g
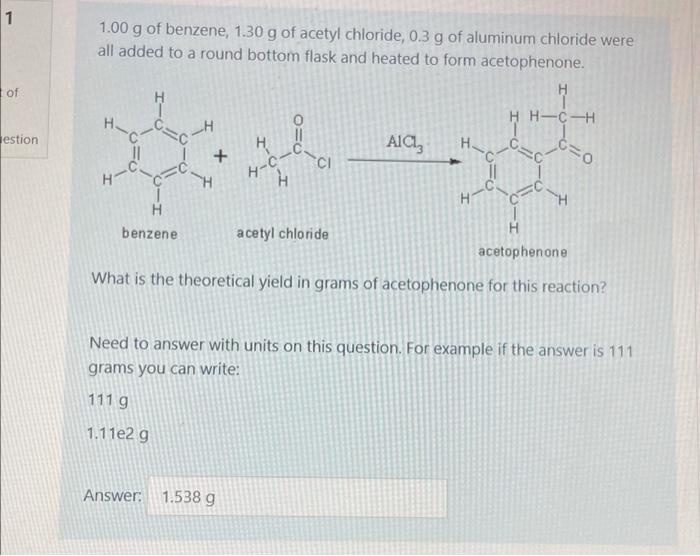
1 1.00 g of benzene, 1.30 g of acetyl chloride, 0.3 g of aluminum chloride were all added to a round bottom flask and heated to form acetophenone. of H H HH-CH H -H estion H Alch + CI H-C- # "H H benzene acetyl chloride H acetophenone What is the theoretical yield in grams of acetophenone for this reaction? Need to answer with units on this question. For example if the answer is 111 grams you can write: 1119 1.11e2 g Answer: 1.538 g
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


