Question: dohe atomic number Based on what basis the elements had been arranged in the modern periodic table? A- The increase in the atomic mass B
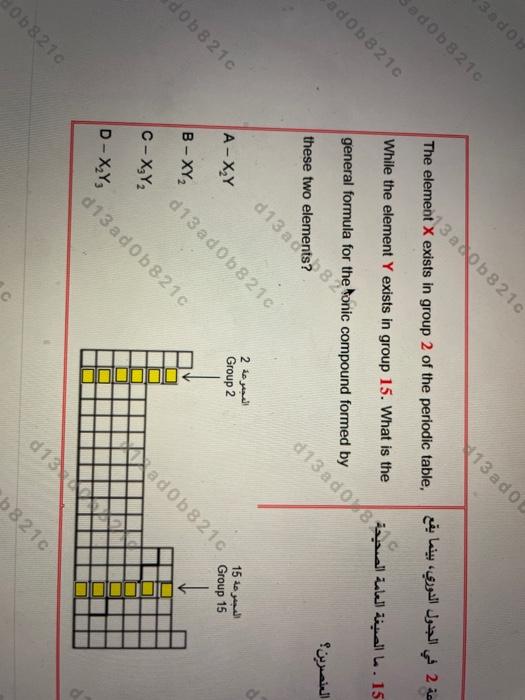


dohe atomic number Based on what basis the elements had been arranged in the modern periodic table? A- The increase in the atomic mass B - The increase d13 ado210 C-The classification of elements into metals and nonmetals D - The classification of elements according to their physical state 210 od 0982162 d 13 ad01210 dob8216 Sodo 13 ado The element X exists in group 2 of the periodic table, 2 adob8210 While the element Y exists in group 15. What is the general formula for the ad0b8210 a these two 2 2 Group 2 15 A-XzY Group 15 B-XY do b 8210 d13 ad0b8210 C-XY2 aad0b8210 JUIN D-XY, d13 a dOb 8210 d13 0b8210 b8210 one 8ic compound formed by 13 a do wwel L. 15 4:31 a 20-3 2021 -2 068210 13 ad0b82 d 13 ad0b82 How the physical properties of iron are altered by d 13 a dOb 8210 forming the carbon steel alloy? A - The iron becomes relatively soft d13 ad0b8210 less hard d13 ad % 8210 C - The iron becomes harder and stronger D - The iron es relatively malleable 13 ad ob 8210 13 ad068218 d 13 a do X8 210 Ob841 B - The iron becomes d13 adob 13 a dob Where is located the element with the electron Poza 53216 d 13 a dOb 8 210 configuration [Kr] 5s 4dlo in the periodic table? d 13 a dOb 8210 A - In period 4 group 12 d 13 ado 72 5 and C - In period 4 group 10 5 and d 13 ad ob 8210 d 13 a do 21 B - In perioa 8.210 D - In perior a 2010 13 on oup 12
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


