Question: Drag conversion units onto the boxes in the equation to make conversions. Some boxes can be left empty. Click on a unit to remove it
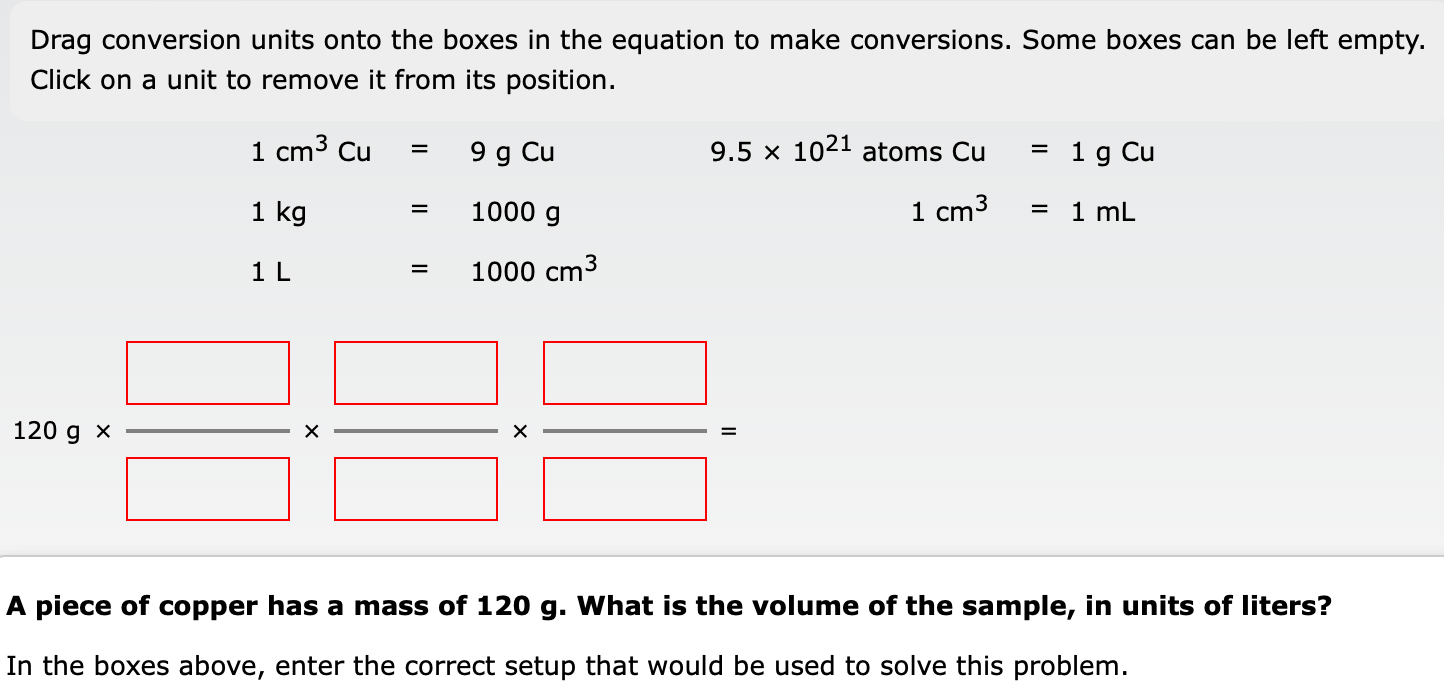
Drag conversion units onto the boxes in the equation to make conversions. Some boxes can be left empty. Click on a unit to remove it from its position. 1cm3Cu1kg1L=9gCu=1000g=1000cm39.51021atomsCu1cm3=1mL=1gCu 120g x = A piece of copper has a mass of 120g. What is the volume of the sample, in units of liters? In the boxes above, enter the correct setup that would be used to solve this
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


