Question: Drag conversion units onto the boxes in the equation to make conversions. Some boxes can be left empty. Click on a unit to remove it
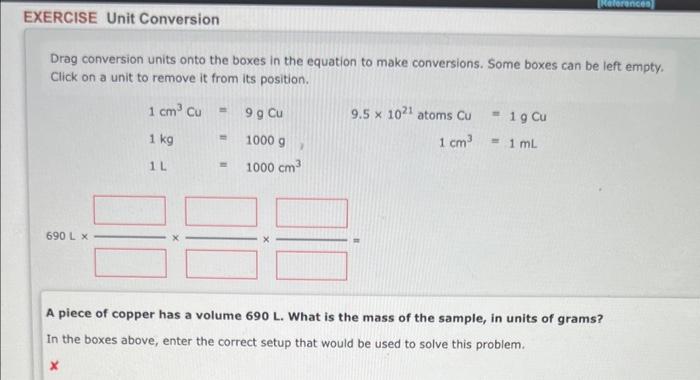
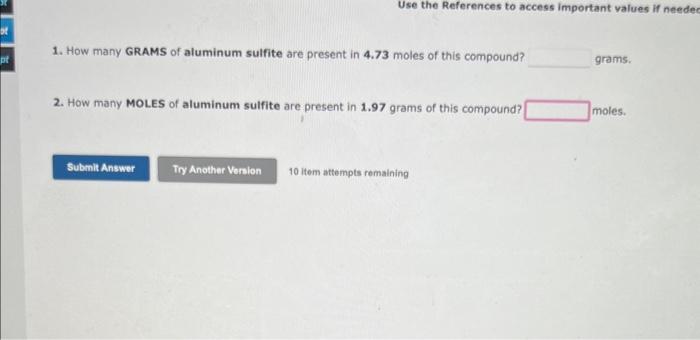
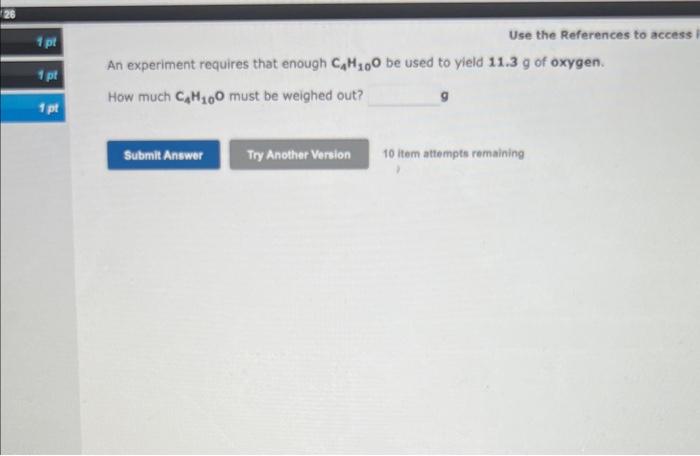
Drag conversion units onto the boxes in the equation to make conversions. Some boxes can be left empty. Click on a unit to remove it from its position. A piece of copper has a volume 690L. What is the mass of the sample, in units of grams? In the boxes above, enter the correct setup that would be used to solve this problem. 1. How many GRAMS of aluminum sulfite are present in 4.73 moles of this compound? grams. 2. How many MOLES of aluminum sulfite are present in 1.97 grams of this compound? moles. An experiment requires that enough C4H10O be used to yield 11.3g of oxygen. How much C4H10O must be weighed out? 10 item attempts remaining
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


