Question: Drag the appropriate elements to their respective bins. A main group metal tends to lose electrons, forming a cation with the samo number of electrons
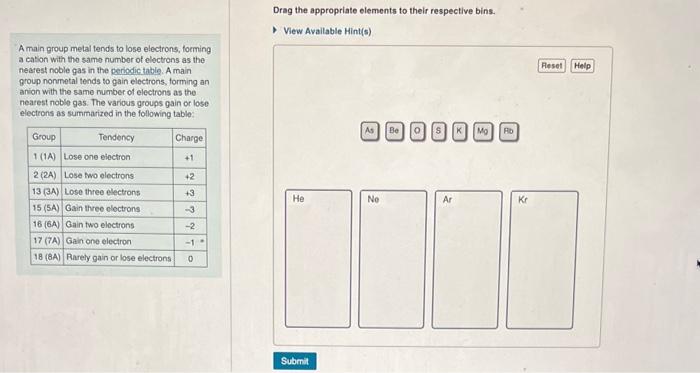
Drag the appropriate elements to their respective bins. A main group metal tends to lose electrons, forming a cation with the samo number of electrons as the nearest noble gas in the periodic table. A main group nonmetal tends to gain electrons, forming an anion with the same number of electrons as the nearest noble gas. The various grougs gain or lose electrons as summarized in the following table
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


