Question: When main group elements react, they tend to acquire eight outer - shell electrons ( n s 2 n p 6 ) . This is
When main group elements react, they tend to acquire eight outershell electrons
This is called an octet, and it is a particularly stable configuration
Nonmetals in the third period and beyond can break the octet rule because they have
vacant orbitals that allow them to accommodate more than eight outershell electrons.
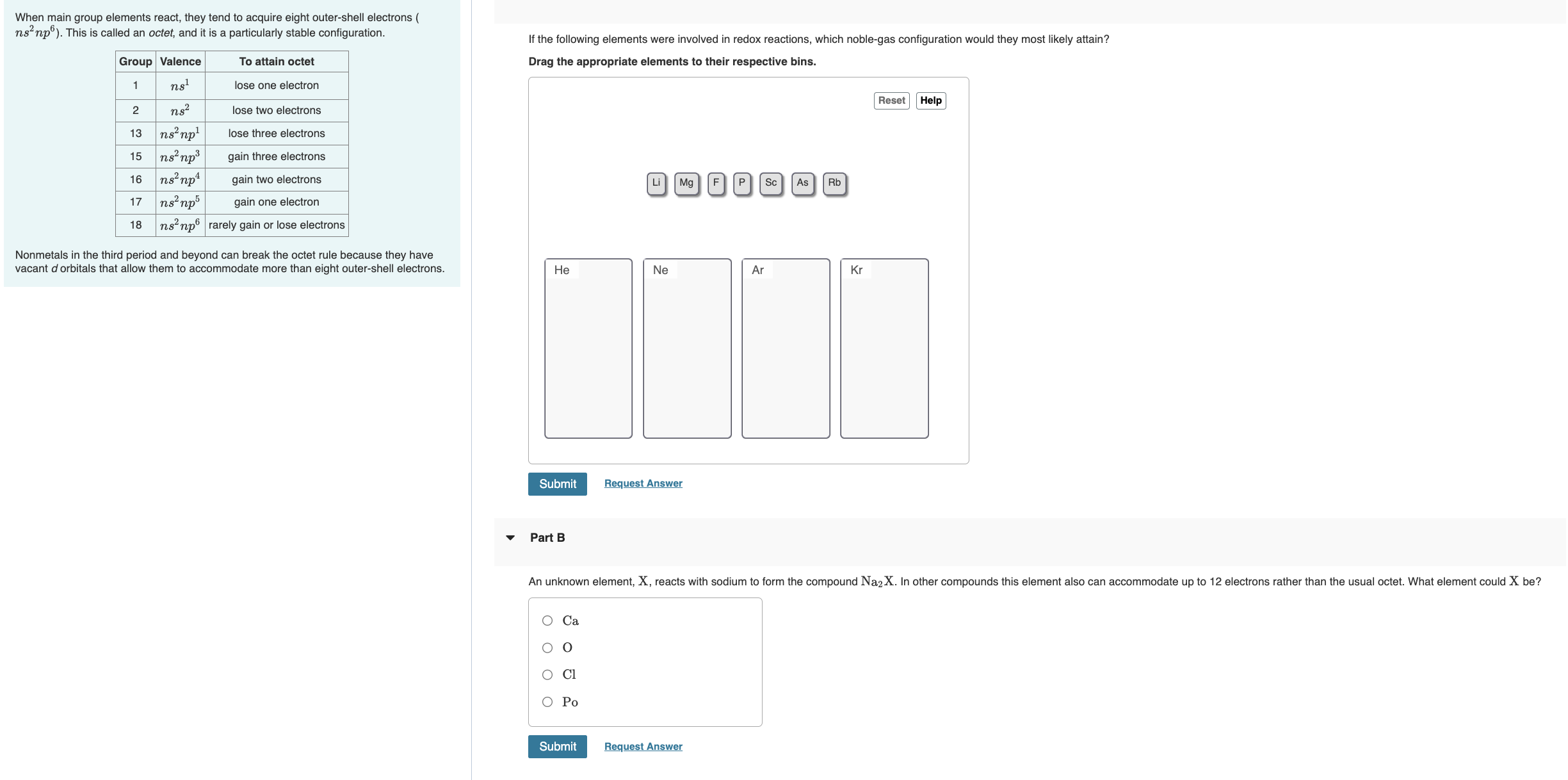
If the following elements were involved in redox reactions, which noblegas configuration would they most likely attain?
Drag the appropriate elements to their respective bins.
Request Answer
Part B
O
Po
Step by Step Solution
There are 3 Steps involved in it
1 Expert Approved Answer
Step: 1 Unlock


Question Has Been Solved by an Expert!
Get step-by-step solutions from verified subject matter experts
Step: 2 Unlock
Step: 3 Unlock


