Question: 1) Draw a Lewis structure for the given skeletal molecule. Note: Multiple bonds and lone pairs are not shown in the skeletal molecule. How many
1) Draw a Lewis structure for the given skeletal molecule.
Note: Multiple bonds and lone pairs are not shown in the skeletal molecule.
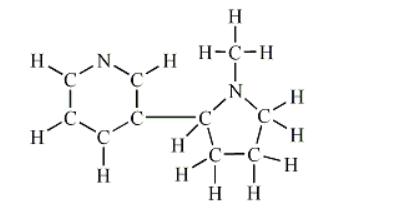
How many C-N bonds are formed by the overlap of a C(sp3) and an N(sp3)?
2) Draw a Lewis structure for the given skeletal molecule.
Note: Multiple bonds and lone pairs are not shown in the skeletal molecule.
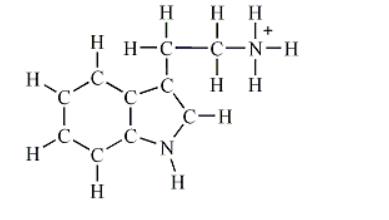
How many lone pairs of electrons are found in the Lewis structure of your molecule?
3) Draw a Lewis structure for the given skeletal molecule.
Note: Multiple bonds and lone pairs are not shown in the skeletal molecule.
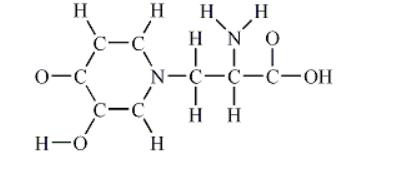
How many π bonds are found in this molecule?
. H I .N, - I I H H H I I -- N / -- | I | H H -
Step by Step Solution
3.44 Rating (151 Votes )
There are 3 Steps involved in it
icr H H H H Lewis Structure N H H ... View full answer

Get step-by-step solutions from verified subject matter experts


