Draw an MO energy diagram for the following - cyclooctatrienyl dication (Use polygon-on corner method... aka Frost
Question:
Draw an MO energy diagram for the following - cyclooctatrienyl dication (Use "polygon-on corner" method... aka Frost circle method). Based on your diagram, which of the following statements is true? 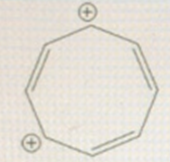 Select one:
Select one:
A. The species contains bonding and antibonding MO's, but no nonbonding MO> are present.
B. All major bonding electronic shells are full and a nonbonding electronic shell contains two unpaired electrons.
C. All major bonding electronic shells are full; there is a nonbonding electronic shell that is empty, and all antibonding MO's are empty. D. All major bonding electronic shells and a nonbonding electronic shell are full all antibonding electronic shells are empty.
E. All major bonding electronic shells and a nonbonding electronic shell are full an antibonding MO has a single, unpaired electron.





