Question: Draw two different Lewis structures for the formula C HGO. Which intermolecular forces occur for each? Which should have the higher boiling point? H H
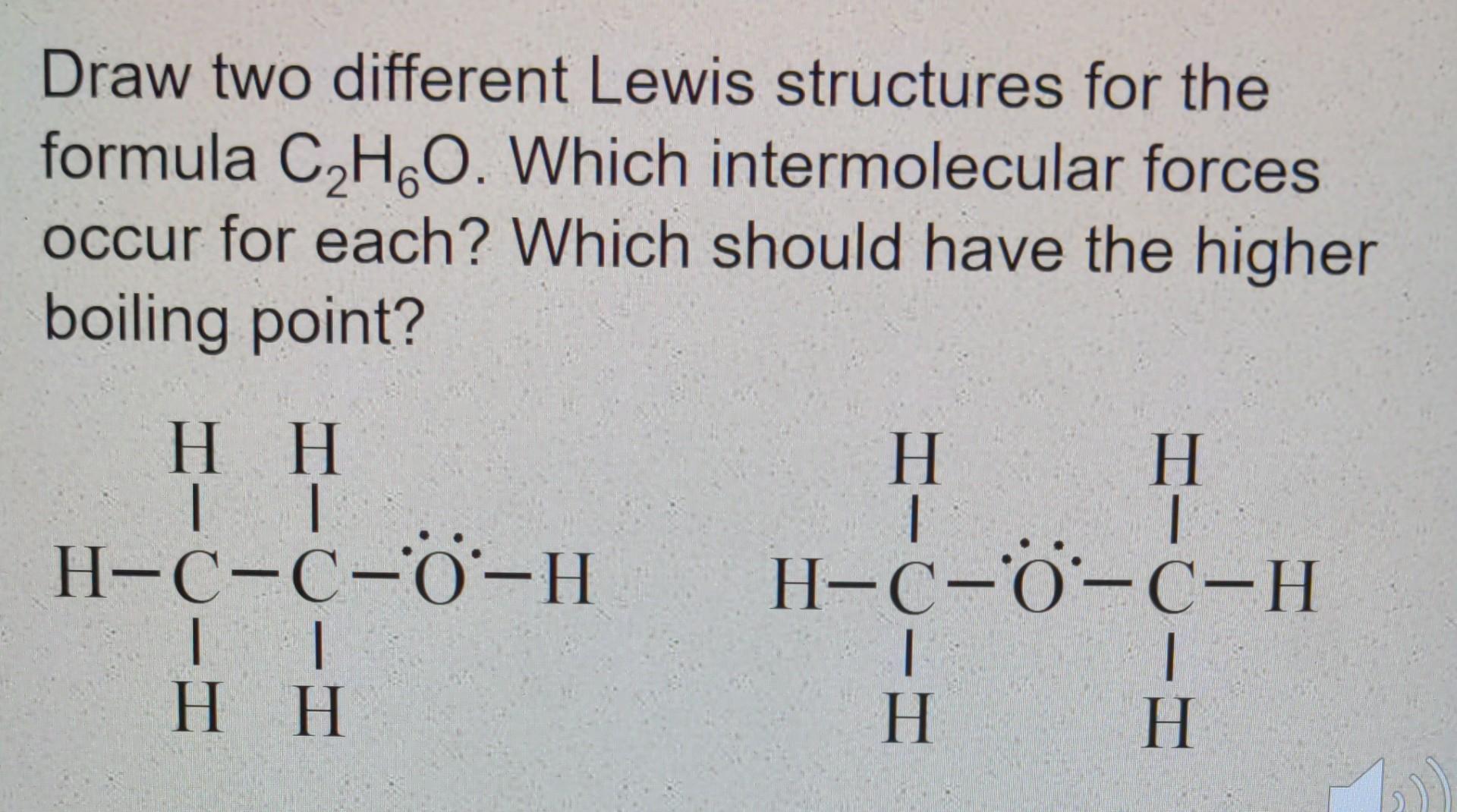

Draw two different Lewis structures for the formula C HGO. Which intermolecular forces occur for each? Which should have the higher boiling point? H H 1 H-0-0-0-H IT H H H H 1 H-C-0-C-H 1 1 H H Consider the following compounds: NH3 CHA H2 Which of the compounds above exhibit London dispersion forces? Which exhibit dipole-dipole forces? Which exhibit hydrogen bonding? Arrange them in order of increasing boiling point
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


