Question: Dry air is used as the O2 source in a combustion reaction. The conversion rate (X) of O2 during the reaction and the reaction rates
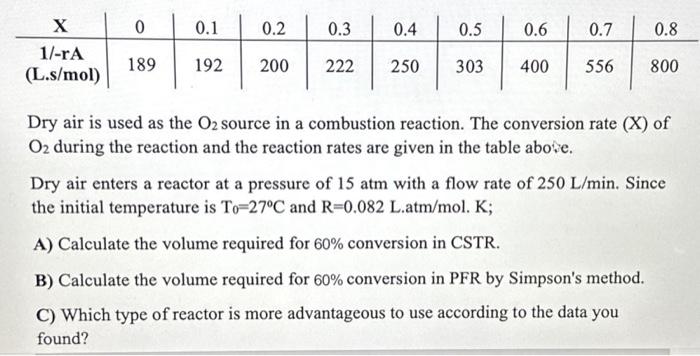
Dry air is used as the O2 source in a combustion reaction. The conversion rate (X) of O2 during the reaction and the reaction rates are given in the table above. Dry air enters a reactor at a pressure of 15 atm with a flow rate of 250 L/min. Since the initial temperature is To-27C and R=0.082 L.atm/mol. K;
A) Calculate the volume required for 60% conversion in CSTR.
B) Calculate the volume required for 60% conversion in PFR by Simpson's method.
C) Which type of reactor is more advantageous to use according to the data you found?
Dry air is used as the O2 source in a combustion reaction. The conversion rate (X) of O2 during the reaction and the reaction rates are given in the table above. Dry air enters a reactor at a pressure of 15atm with a flow rate of 250L/min. Since the initial temperature is T0=27C and R=0.082L.atm/mol.K; A) Calculate the volume required for 60% conversion in CSTR. B) Calculate the volume required for 60% conversion in PFR by Simpson's method. C) Which type of reactor is more advantageous to use according to the data you found
Step by Step Solution
There are 3 Steps involved in it
1 Expert Approved Answer
Step: 1 Unlock


Question Has Been Solved by an Expert!
Get step-by-step solutions from verified subject matter experts
Step: 2 Unlock
Step: 3 Unlock


