Question: During the nickel-plating process, a solution containing nickel is applied to the mechanical parts which need to be plated, followed by rinsing of the parts
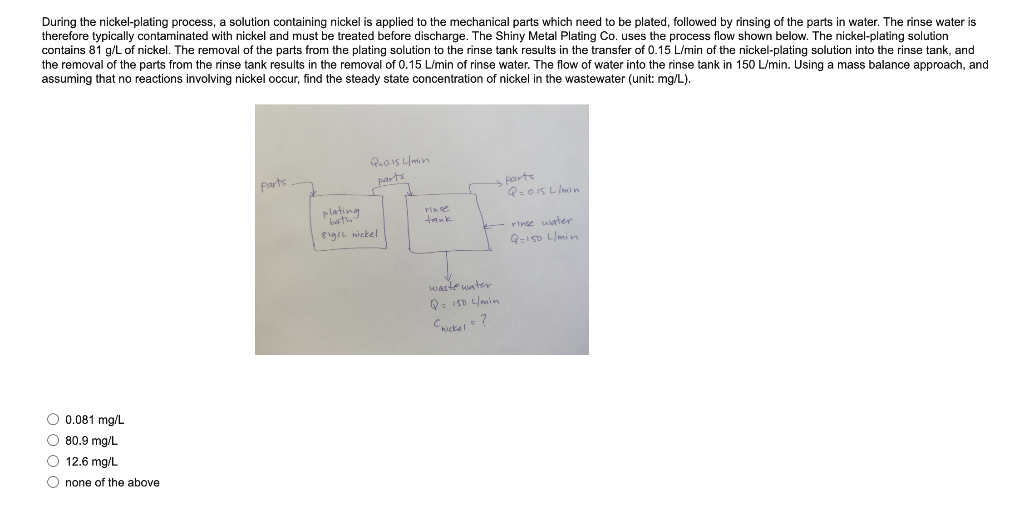
During the nickel-plating process, a solution containing nickel is applied to the mechanical parts which need to be plated, followed by rinsing of the parts in water. The rinse water is therefore typically contaminated with nickel and must be treated before discharge. The Shiny Metal Plating Co. uses the process flow shown below. The nickel-plating solution contains 81g/L of nickel. The removal of the parts from the plating solution to the rinse tank results in the transfer of 0.15L/min of the nickel-plating solution into the rinse tank, and the removal of the parts from the rinse tank results in the removal of 0.15L/min of rinse water. The flow of water into the rinse tank in 150L/min. Using a mass balance approach, and assuming that no reactions involving nickel occur, find the steady state concentration of nickel in the wastewater (unit: mg/L). 0.081mg/L80.9mg/L12.6mg/L none of the above
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


