Question: e) Consider the below reaction 2A+BC+DwhereIAA=kCAACB where CA and CEE are concentrations of reactant A and B If CAO=0.024mol/L and CBOB=0.036mol/L, calculate XACB, and CC
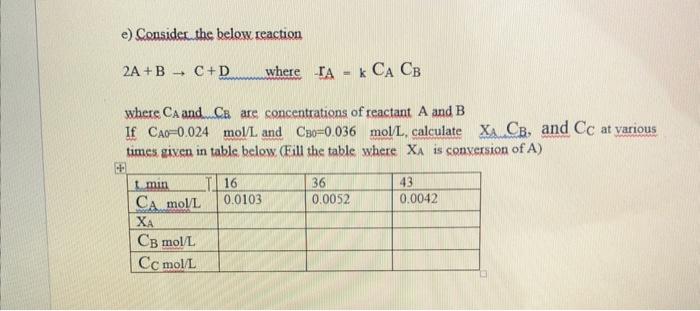
e) Consider the below reaction 2A+BC+DwhereIAA=kCAACB where CA and CEE are concentrations of reactant A and B If CAO=0.024mol/L and CBOB=0.036mol/L, calculate XACB, and CC at various times given in table below (Fill the table where XA is conversion of A )
Step by Step Solution
There are 3 Steps involved in it
1 Expert Approved Answer
Step: 1 Unlock


Question Has Been Solved by an Expert!
Get step-by-step solutions from verified subject matter experts
Step: 2 Unlock
Step: 3 Unlock


