Question: e reaction between C and D is represented as follows: C+DE The experiment results are shown in the table below: i. Calculate the rate of
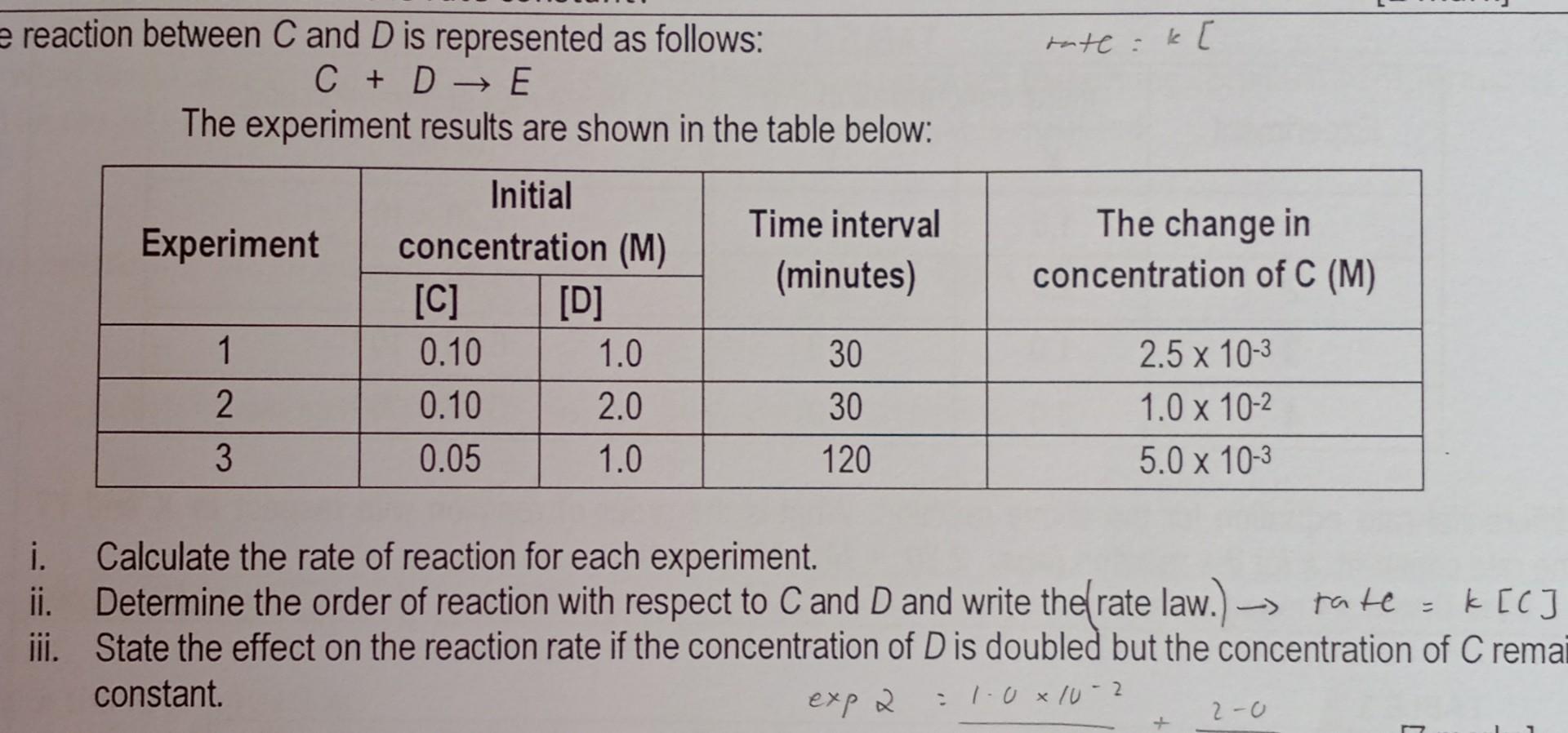
e reaction between C and D is represented as follows: C+DE The experiment results are shown in the table below: i. Calculate the rate of reaction for each experiment. ii. Determine the order of reaction with respect to C and D and write the (rate law.) tatC=k[C] iii. State the effect on the reaction rate if the concentration of D is doubled but the concentration of C reme constant
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


