Question: e) The table below lists calculated crystal field activation energies (CFAE) in units of Dq (oct=10Dq) as a function of their d-electron counts (dn) for
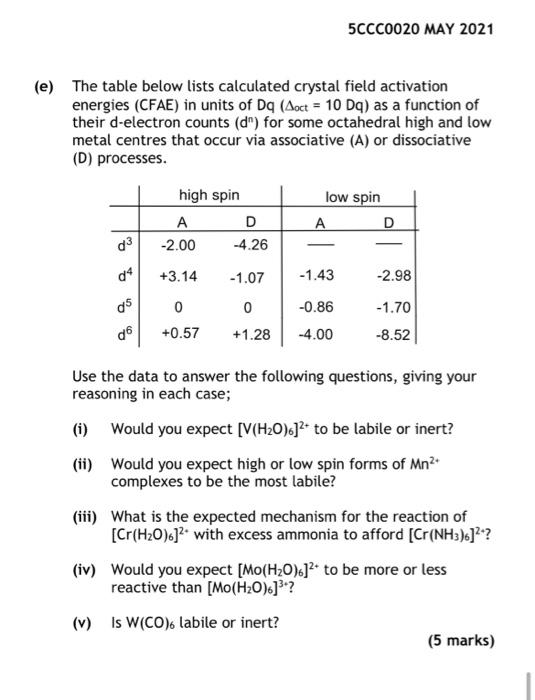
e) The table below lists calculated crystal field activation energies (CFAE) in units of Dq (oct=10Dq) as a function of their d-electron counts (dn) for some octahedral high and low metal centres that occur via associative (A) or dissociative (D) processes. Use the data to answer the following questions, giving your reasoning in each case; (i) Would you expect [V(H2O)6]2+ to be labile or inert? (ii) Would you expect high or low spin forms of Mn2+ complexes to be the most labile? (iii) What is the expected mechanism for the reaction of [Cr(H2O)6]2+ with excess ammonia to afford [Cr(NH3)6]2+ ? (iv) Would you expect [Mo(H2O)6]2+ to be more or less reactive than [Mo(H2O)6]3+ ? (v) Is W(CO)6 labile or inert
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


