Question: Each of the three following processes transforms a system from a thermodynamic equilibrium state 1 to a different thermodynamic equilibrium state 2. For each of
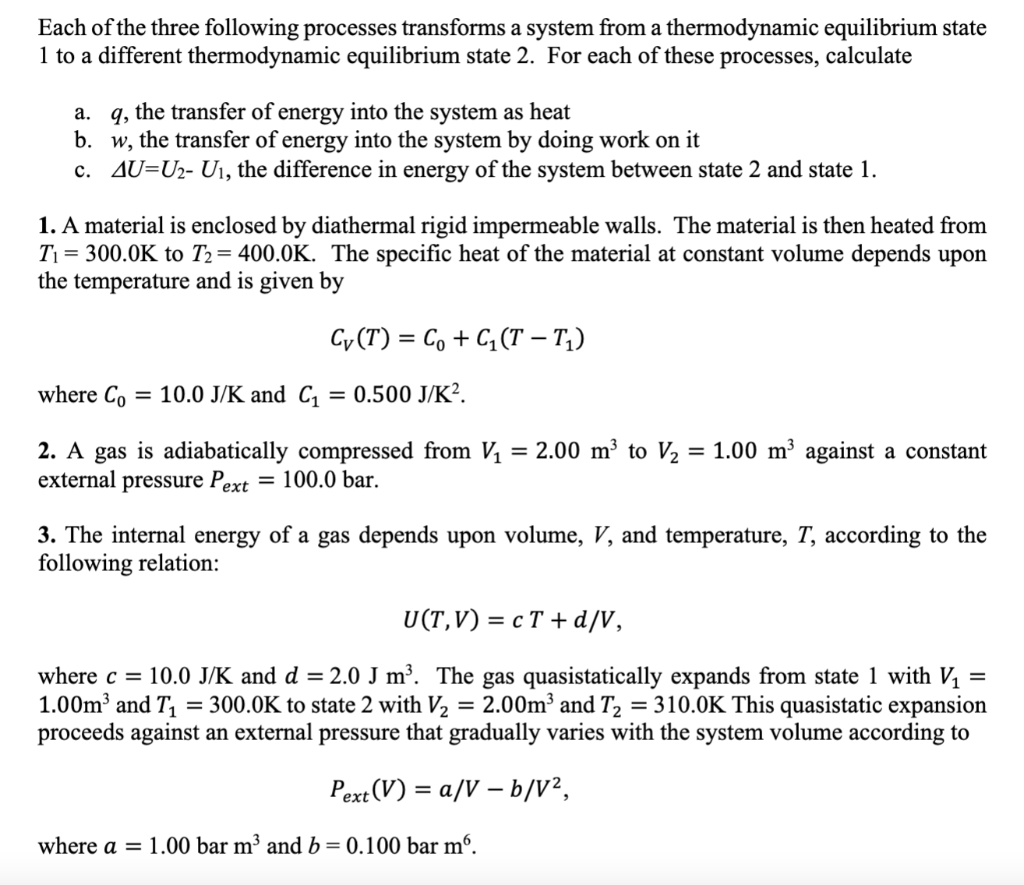
Each of the three following processes transforms a system from a thermodynamic equilibrium state 1 to a different thermodynamic equilibrium state 2. For each of these processes, calculate a. q, the transfer of energy into the system as heat b. w, the transfer of energy into the system by doing work on it c. AU=U2- U, the difference in energy of the system between state 2 and state 1. 1. A material is enclosed by diathermal rigid impermeable walls. The material is then heated from T = 300.0K to T2 = 400.0K. The specific heat of the material at constant volume depends upon the temperature and is given by Cy(T) = Co + C7 (T T;) where Co = 10.0 J/K and C1 = 0.500 J/K2. = 2. A gas is adiabatically compressed from V2 = 2.00 m to 12 = 1.00 m against a constant external pressure Pext = 100.0 bar. 3. The internal energy of a gas depends upon volume, V, and temperature, T, according to the following relation: U(T,V) = c T +d/V, = where c = 10.0 J/K and d = 2.0 J m. The gas quasistatically expands from state 1 with V2 = 1.00m and T1 = 300.0K to state 2 with V2 = 2.00m and T2 = 310.0K This quasistatic expansion proceeds against an external pressure that gradually varies with the system volume according to = Pext(V) = a/V b/V?, = where a = 1.00 bar m and b= 0.100 bar m
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


