Question: Each row of the table below describes an aqueous solution at 25 C. The second column of the table shows the initial components of the
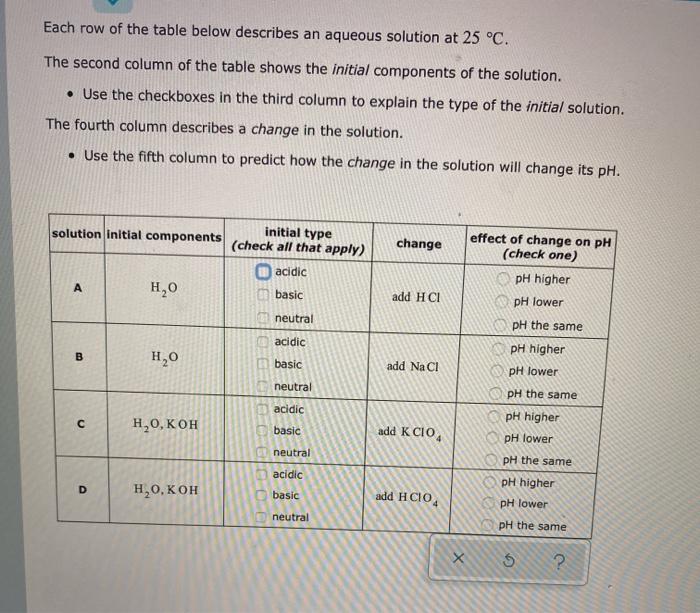
Each row of the table below describes an aqueous solution at 25 C. The second column of the table shows the initial components of the solution. Use the checkboxes in the third column to explain the type of the initial solution. The fourth column describes a change in the solution. Use the fifth column to predict how the change in the solution will change its pH. initial type solution initial components (check all that apply) change acidic basic H2O add HCI effect of change on pH (check one) pH higher pH lower pH the same pH higher neutral acidic B HO basic add NaCl pH lower neutral pH the same ET GOOGLE C acidic pH higher ,0, basic add K CIO pH lower neutral acidic pH the same pH higher pH lower D , basic add HCIO neutral pH the same 5
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


