Question: Each row of the table below describes an aqueous solution at 25 C. The second column of the table shows the initial components of the
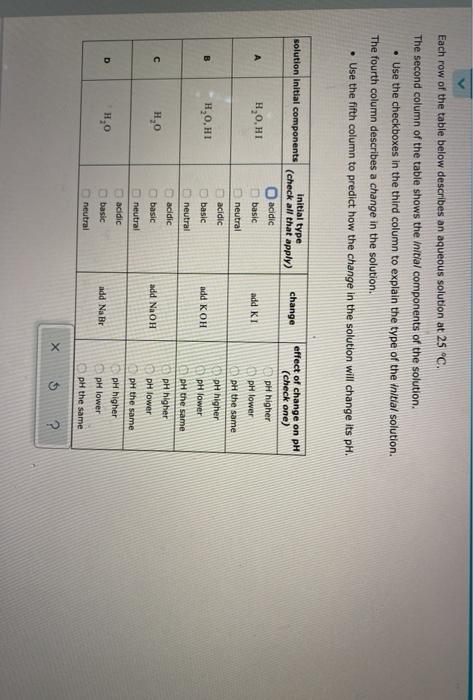
Each row of the table below describes an aqueous solution at 25 C. The second column of the table shows the initial components of the solution Use the checkboxes in the third column to explain the type of the initial solution. The fourth column describes a change in the solution. Use the fifth column to predict how the change in the solution will change its pH. solution initial components change initial type (check all that apply) acidic basic effect of change on pH (check one) pH higher H,0,HI add KI pH lower neutral pH the same pH higher pH lower B acidic basic H,O,H1 add KOH pH the same neutral acidic basic pH higher 1,0 add NaOH pH lower neutral pH the same pH higher D acidic basic 1,0 add NaBr pH lower neutral pH the same
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


