Question: that's all the information provided. Using the tables, what is the standard entropy change for the following reaction? CH4(g)+2O2(g)CO2(g)+2H2O(g) Report your answer in units of
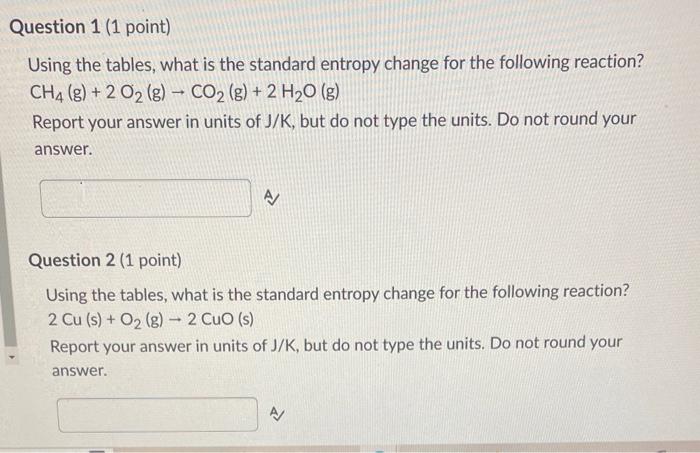

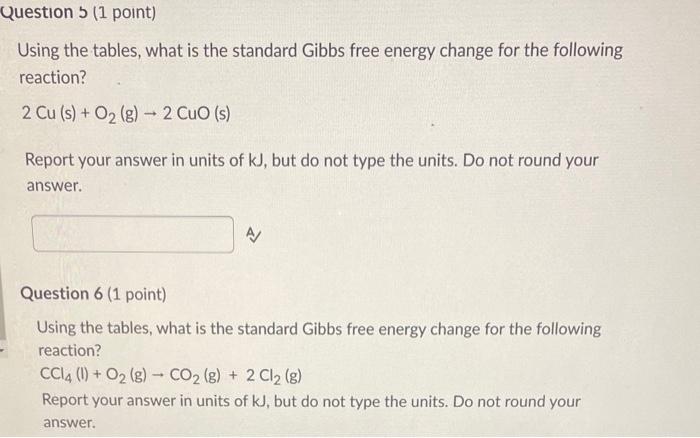
Using the tables, what is the standard entropy change for the following reaction? CH4(g)+2O2(g)CO2(g)+2H2O(g) Report your answer in units of J/K, but do not type the units. Do not round your answer. Question 2 (1 point) Using the tables, what is the standard entropy change for the following reaction? 2Cu(s)+O2(g)2CuO(s) Report your answer in units of J/K, but do not type the units. Do not round your answer. Using the tables, what is the standard entropy change for the following reaction? CCl4(l)+O2(g)CO2(g)+2Cl2(g) Report your answer in units of J/K, but do not type the units. Do not round your answer. Question 4 (1 point) Using the tables, what is the standard Gibbs free energy change for the following reaction? CH4(g)+2O2(g)CO2(g)+2H2O(g) Report your answer in units of kJ, but do not type the units. Do not round your answer. Using the tables, what is the standard Gibbs free energy change for the following reaction? 2Cu(s)+O2(g)2CuO(s) Report your answer in units of kJ, but do not type the units. Do not round your answer. Question 6 (1 point) Using the tables, what is the standard Gibbs free energy change for the following reaction? CCl4(l)+O2(g)CO2(g)+2Cl2(g) Report your answer in units of kJ, but do not type the units. Do not round your
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


