Question: Eight Erlenmeyer flasks were filled with 2 5 0 ml of solution containing about 5 0 0 mg / L of pesticide. The flasks were
Eight Erlenmeyer flasks were filled with ml of solution containing about mgL of pesticide. The flasks were dosed with various weights of powdered activated carbon. Samples taken from flasks filtered to remove carbon and pesticide in filtrate was analyzed after equilibrium conditions were reached.a Determine the relationship of adsorption with pesticide concentration. Which isotherm gives a better fit to the data provided?b Using isotherm found in part a determine the mass of activated carbon in batch process to remove of pesticide from mL of water with mgL pesticide initial concentration.
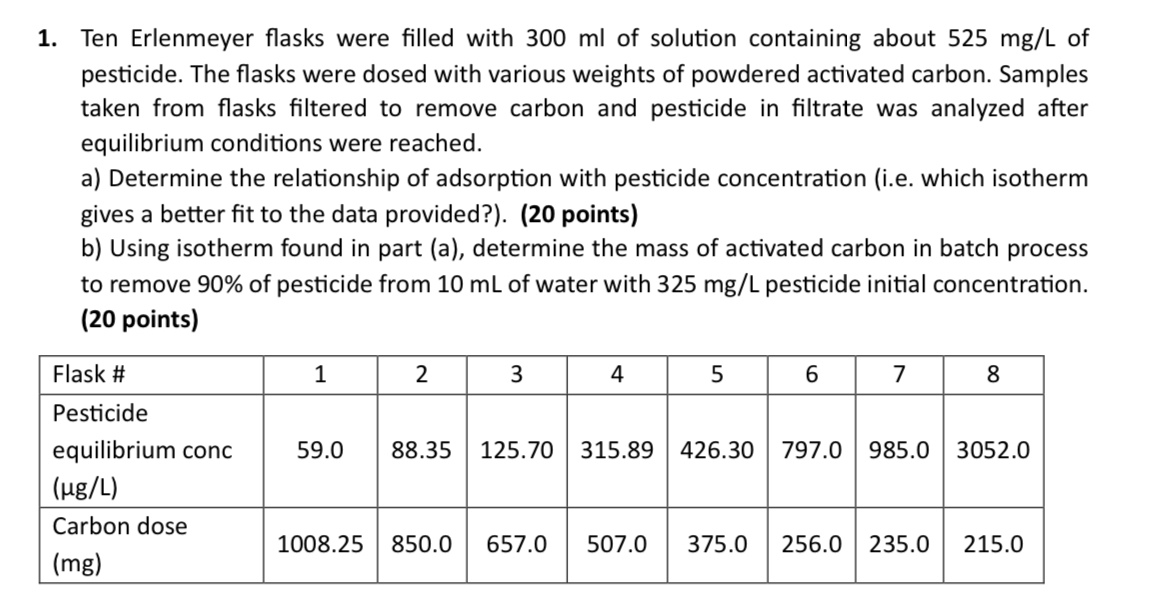
Ten Erlenmeyer flasks were filled with of solution containing about of pesticide. The flasks were dosed with various weights of powdered activated carbon. Samples taken from flasks filtered to remove carbon and pesticide in filtrate was analyzed after equilibrium conditions were reached.
a Determine the relationship of adsorption with pesticide concentration ie which isotherm gives a better fit to the data provided? points
b Using isotherm found in part a determine the mass of activated carbon in batch process to remove of pesticide from of water with pesticide initial concentration. points
tableFlask #tablePesticideequilibrium conc
Step by Step Solution
There are 3 Steps involved in it
1 Expert Approved Answer
Step: 1 Unlock


Question Has Been Solved by an Expert!
Get step-by-step solutions from verified subject matter experts
Step: 2 Unlock
Step: 3 Unlock


