Question: Electrolytes exist in water as separate ions, which causes the solution to conduct electricity. Nonelectrolytes are generally molecular compounds that remain as molecules when dissolved
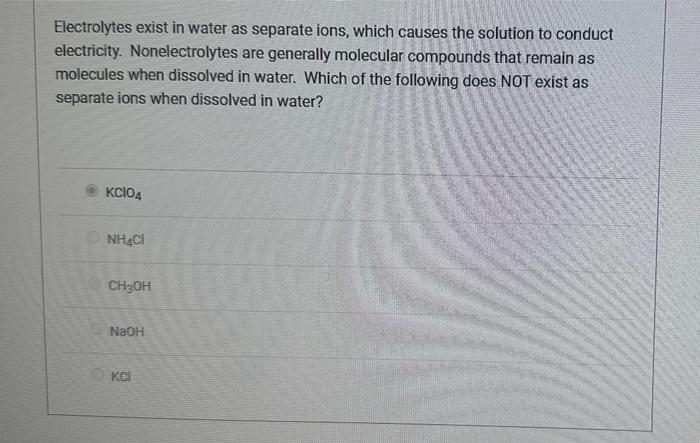
Electrolytes exist in water as separate ions, which causes the solution to conduct electricity. Nonelectrolytes are generally molecular compounds that remain as molecules when dissolved in water. Which of the following does NOT exist as separate ions when dissolved in water? KClO4 NH4Cl CH3OH NaOH
Step by Step Solution
There are 3 Steps involved in it
1 Expert Approved Answer
Step: 1 Unlock


Question Has Been Solved by an Expert!
Get step-by-step solutions from verified subject matter experts
Step: 2 Unlock
Step: 3 Unlock


