Question: Electron Configurations and the Periodic Table In the periodic table elements are organized by atomic number, the number of protons in an atom. The
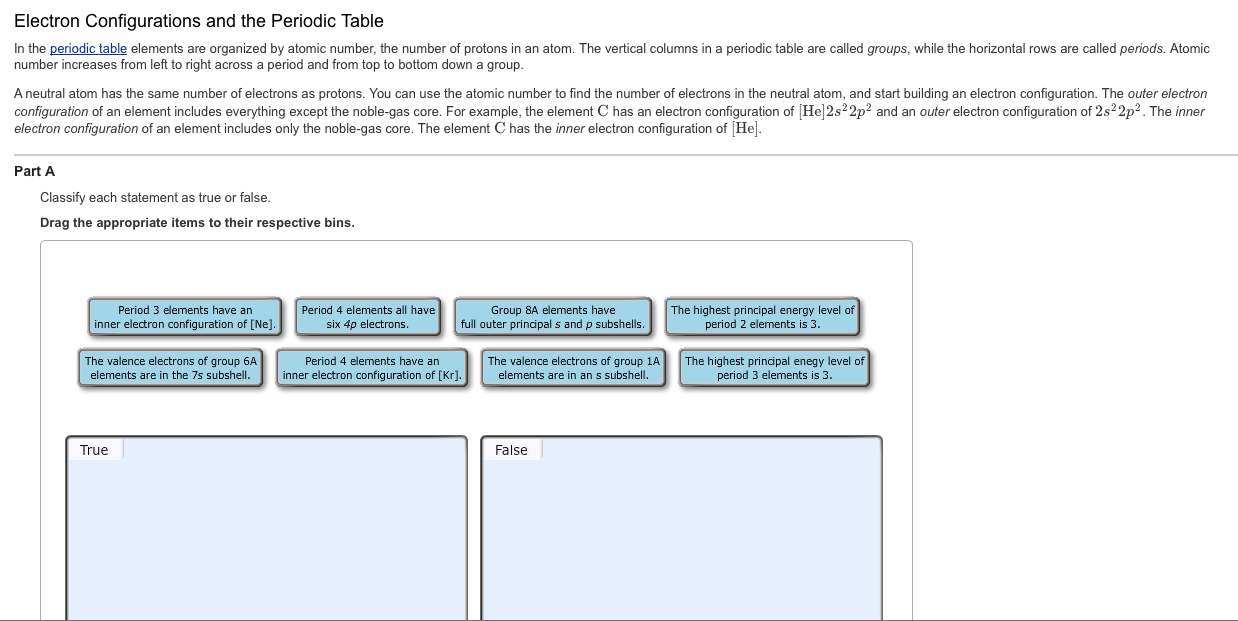
Electron Configurations and the Periodic Table In the periodic table elements are organized by atomic number, the number of protons in an atom. The vertical columns in a periodic table are called groups, while the horizontal rows are called periods. Atomic number increases from left to right across a period and from top to bottom down a group. A neutral atom has the same number of electrons as protons. You can use the atomic number to find the number of electrons in the neutral atom, and start building an electron configuration. The outer electron configuration of an element includes everything except the noble-gas core. For example, the element C has an electron configuration of [He]2s22p and an outer electron configuration of 2s22p. The inner electron configuration of an element includes only the noble-gas core. The element C has the inner electron configuration of [He]. Part A Classify each statement as true or false. Drag the appropriate items to their respective bins. Period 3 elements have an inner electron configuration of [Ne]. The valence electrons of group 6A elements are in the 7s subshell. True Period 4 elements all have. six 4p electrons. Group 8A elements have full outer principal s and p subshells Period 4 elements have an inner electron configuration of [Kr]. The valence electrons of group 1A elements are in an s subshell. False The highest principal energy level of period 2 elements is 3. The highest principal enegy level of period 3 elements is 3.
Step by Step Solution
3.36 Rating (152 Votes )
There are 3 Steps involved in it
The detailed answer for the above question is provided below Period 3 elements have an inner electro... View full answer

Get step-by-step solutions from verified subject matter experts


