Question: Electron Structure 1. Draw the Bohr model for hydrogen with a single electron. Show the n1,n2,n3 energy levels with the electron in the first or
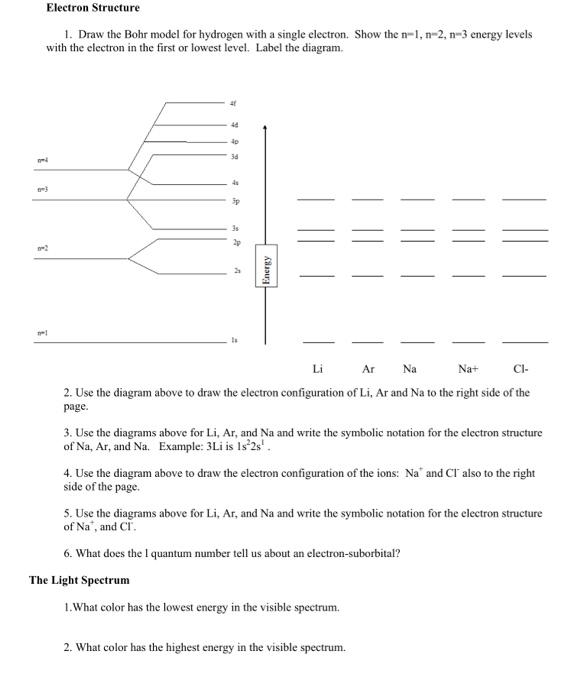

Electron Structure 1. Draw the Bohr model for hydrogen with a single electron. Show the n1,n2,n3 energy levels with the electron in the first or lowest level. Label the diagram. 2. Use the diagram above to draw the electron configuration of Li, Ar and Na to the right side of the page. 3. Use the diagrams above for Li,Ar, and Na and write the symbolic notation for the electron structure of Na,Ar, and Na. Example: 3Li is 1s22s4. 4. Use the diagram above to draw the electron configuration of the ions: Na+and Clalso to the right side of the page. 5. Use the diagrams above for Li,Ar, and Na and write the symbolic notation for the electron structure of Na+, and Cl. 6. What does the I quantum number tell us about an electron-suborbital? The Light Spectrum 1. What color has the lowest energy in the visible spectrum. 2. What color has the highest energy in the visible spectrum. 3. What is the spectrum called at lower energies than red. 4. What is the spectrum called at higher energies than violet
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


