Question: Enter electrons as: e. Use smallest possible integer coefficients. if ab bois is not needed, leave it blznk. For the following electron-transter reaction: 2Cr(s)+3N)2+(aq)2Cr3+(aq)+3Ni(s) The
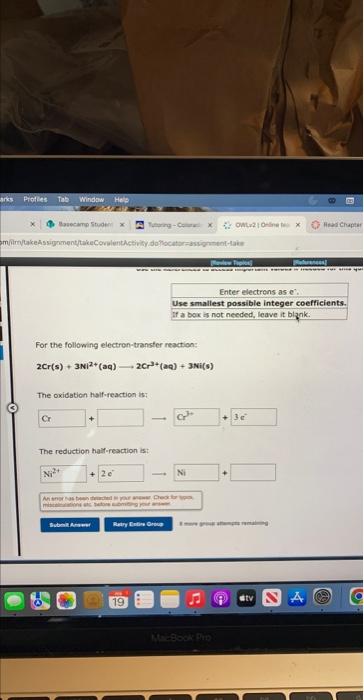
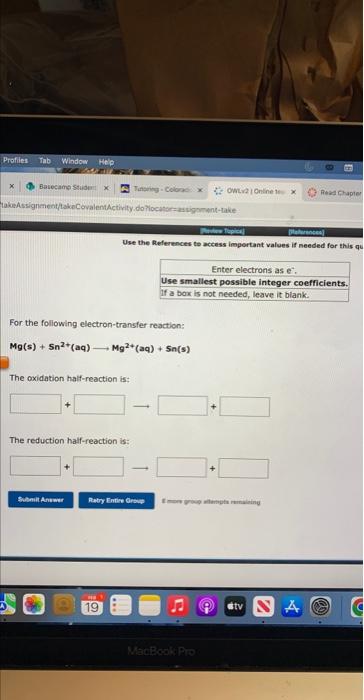
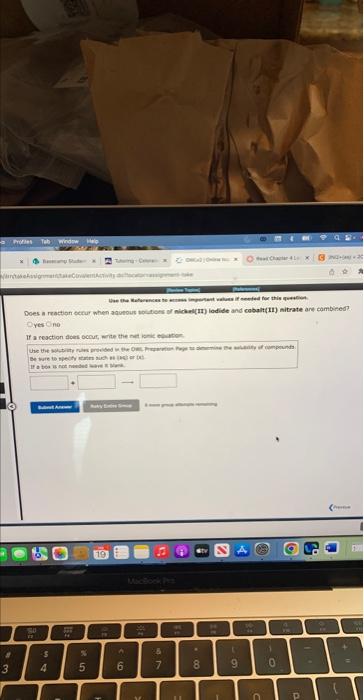
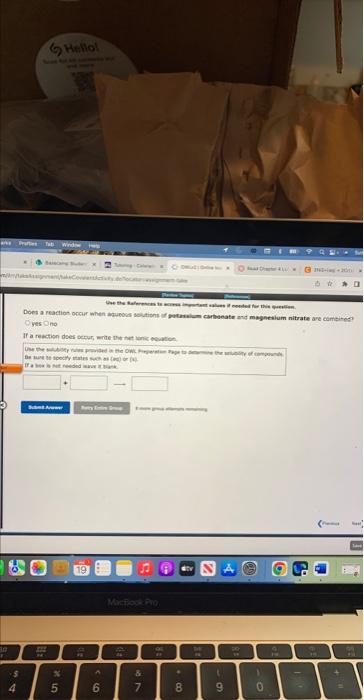
Enter electrons as: e. Use smallest possible integer coefficients. if ab bois is not needed, leave it blznk. For the following electron-transter reaction: 2Cr(s)+3N)2+(aq)2Cr3+(aq)+3Ni(s) The oxidation half-reaction is? The reduction haif-resction ist Use the Peferences te actess important values if needed for this 4 For the following electron-transfer reaction: Mg(s)+5n2+(aq)Mg2+(aq)+5s(s) The oxidation half-reaction is: The reduction half-reaction is: yes no If a cooction alces ocour, werte the net iemic etwiber. Be thare to tieify rartin airh oi lusb er tei yins and If a reaction dees becur, nite the thet lanic esubtion
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


