Question: Enter the quantities for each chemical in the table below Mg(OH)2 (product) MgCl2 (reactant 1) NaOH (reactant 2) Mass (g) 2.00 Molar mass (g/mol) Moles
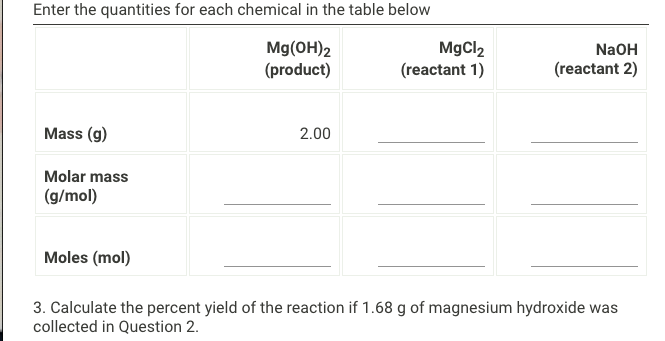
Enter the quantities for each chemical in the table below Mg(OH)2 (product) MgCl2 (reactant 1) NaOH (reactant 2) Mass (g) 2.00 Molar mass (g/mol) Moles (mol) 3. Calculate the percent yield of the reaction if 1.68 g of magnesium hydroxide was collected in Question 2
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


