Question: Help for part 2 especially %yield limiting reactant and second table and equation for synthesis reaction. If all my questions are answered i will give
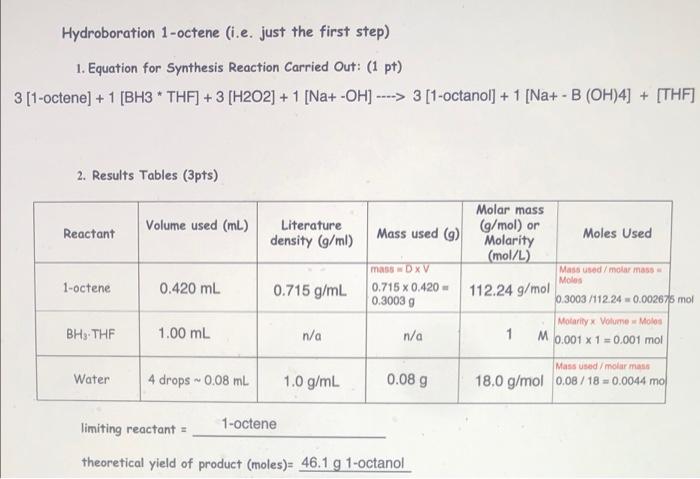
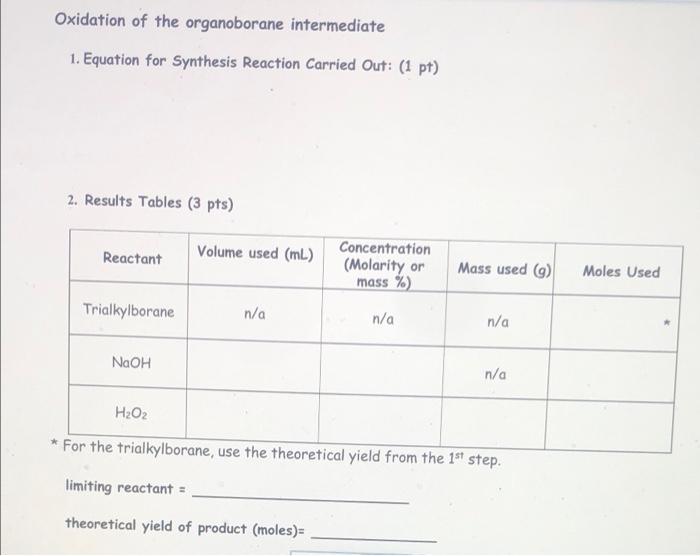
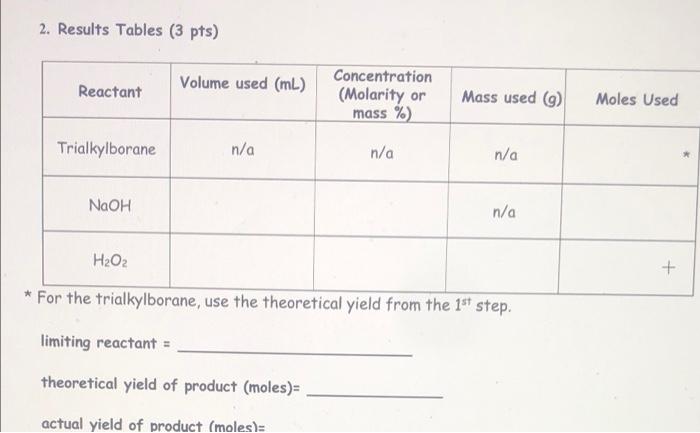
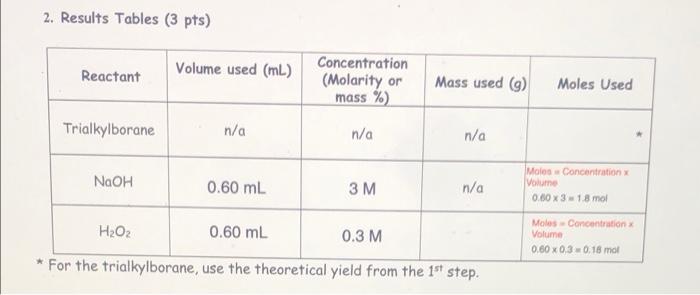
Hydroboration 1-octene (i.e. just the first step) 1. Equation for Synthesis Reaction Carried out: (1 pt) 3 [1-octene) + 1 (BH3 * THF] + 3 (H2O2) + 1 [Na+ -OH) ----> 3 [1-octanol] + 1 [Na+ - B (OH)4] + [THF] - 2. Results Tables (3pts) Reactant Volume used (mL.) Literature density (g/ml) 1-octene 0.420 ml 0.715 g/mL Molar mass (g/mol) or Mass used (9) Molarity Moles Used (mol/L) mas DxV Mass used/molur mass Moles 0.715 x 0.420 - 112.24 g/mol 0.30039 0.3003 /112.24 0.002675 mol Motarilyx Volume - Molos n/a 1 M 0.001 x 1 = 0.001 mol Mass used/moler mas 0.08 g 18.0 g/mol 0.08/18 = 0.0044 mo BHS THE 1.00 mL n/a Water 4 drops 0.08 ml 1.0 g/mL limiting reactant = 1-octene theoretical yield of product (moles)= 46.1 g 1-octanol Oxidation of the organoborane intermediate 1. Equation for Synthesis Reaction Carried Out: (1 pt) 2. Results Tables (3 pts) Reactant Volume used (mL.) Concentration (Molarity or mass %) Mass used (9) Moles Used Trialkylborane n/a n/a n/a NaOH n/a H2O2 * For the trialkylborane, use the theoretical yield from the 1st step. limiting reactant = theoretical yield of product (moles)= 2. Results Tables (3 pts) Reactant Volume used (mL) Concentration (Molarity or mass %) Mass used (9) Moles Used Trialkylborane n/a n/a n/a NaOH n/a HO + * For the trialkylborane, use the theoretical yield from the 1st step. limiting reactant = theoretical yield of product (moles) actual yield of product (moles): 2. Results Tables (3 pts) Reactant Volume used (mL) Concentration (Molarity or mass %) Mass used ) Moles Used Trialkylborane n/a n/a n/a NaOH 0.60 mL 3M n/a Mole Concentration Volume 0.60 x 3 18 mol Moles - Concentration Volume 0.60 0.3 0.18 mot H2O2 0.60 mL 0.3 M * For the trialkylborane, use the theoretical yield from the 1st step. Hydroboration 1-octene (i.e. just the first step) 1. Equation for Synthesis Reaction Carried out: (1 pt) 3 [1-octene) + 1 (BH3 * THF] + 3 (H2O2) + 1 [Na+ -OH) ----> 3 [1-octanol] + 1 [Na+ - B (OH)4] + [THF] - 2. Results Tables (3pts) Reactant Volume used (mL.) Literature density (g/ml) 1-octene 0.420 ml 0.715 g/mL Molar mass (g/mol) or Mass used (9) Molarity Moles Used (mol/L) mas DxV Mass used/molur mass Moles 0.715 x 0.420 - 112.24 g/mol 0.30039 0.3003 /112.24 0.002675 mol Motarilyx Volume - Molos n/a 1 M 0.001 x 1 = 0.001 mol Mass used/moler mas 0.08 g 18.0 g/mol 0.08/18 = 0.0044 mo BHS THE 1.00 mL n/a Water 4 drops 0.08 ml 1.0 g/mL limiting reactant = 1-octene theoretical yield of product (moles)= 46.1 g 1-octanol Oxidation of the organoborane intermediate 1. Equation for Synthesis Reaction Carried Out: (1 pt) 2. Results Tables (3 pts) Reactant Volume used (mL.) Concentration (Molarity or mass %) Mass used (9) Moles Used Trialkylborane n/a n/a n/a NaOH n/a H2O2 * For the trialkylborane, use the theoretical yield from the 1st step. limiting reactant = theoretical yield of product (moles)= 2. Results Tables (3 pts) Reactant Volume used (mL) Concentration (Molarity or mass %) Mass used (9) Moles Used Trialkylborane n/a n/a n/a NaOH n/a HO + * For the trialkylborane, use the theoretical yield from the 1st step. limiting reactant = theoretical yield of product (moles) actual yield of product (moles): 2. Results Tables (3 pts) Reactant Volume used (mL) Concentration (Molarity or mass %) Mass used ) Moles Used Trialkylborane n/a n/a n/a NaOH 0.60 mL 3M n/a Mole Concentration Volume 0.60 x 3 18 mol Moles - Concentration Volume 0.60 0.3 0.18 mot H2O2 0.60 mL 0.3 M * For the trialkylborane, use the theoretical yield from the 1st step
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


