Question: Enter your answer in the provided box. A 40.5-g sample of dinitrogen monoxide is confined in a 4.06L vessel. What is the pressure (in atm)
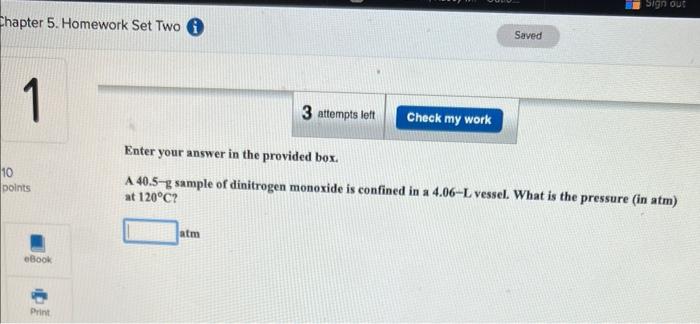
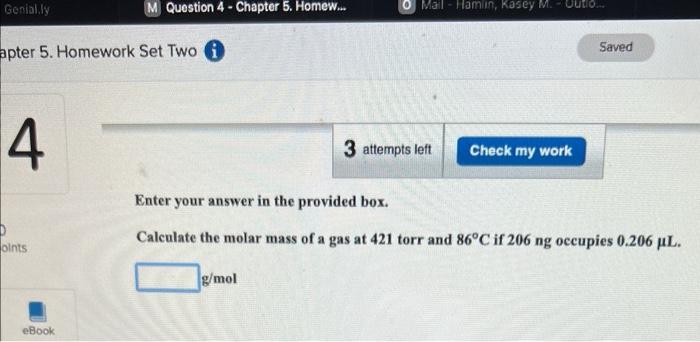
Enter your answer in the provided box. A 40.5-g sample of dinitrogen monoxide is confined in a 4.06L vessel. What is the pressure (in atm) at 120C ? atm Enter your answer in the provided box. Calculate the molar mass of a gas at 421 torr and 86C if 206ng occupies 0.206L. g/mol
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


