Question: Enter your answer in the provided box. A typical reaction between an antacid and the hydrochloric acid in gastric juice is NaHCO3(s)+HCl(aq)NaCl(aq)+H2O(l)+CO2(g) Calculate the volume
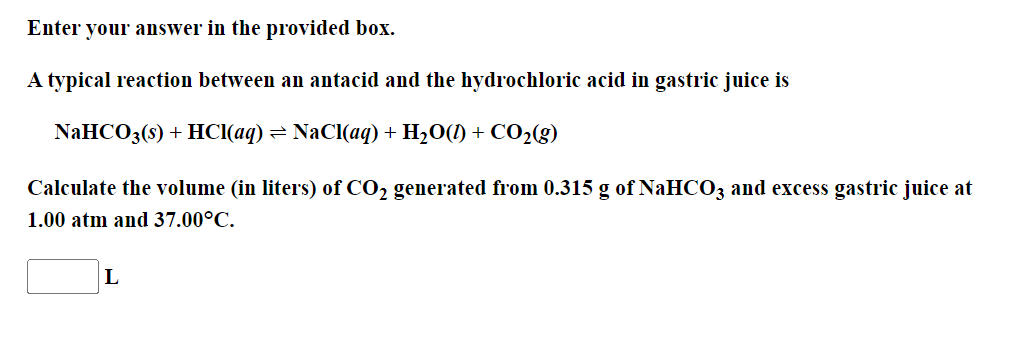
Enter your answer in the provided box. A typical reaction between an antacid and the hydrochloric acid in gastric juice is NaHCO3(s)+HCl(aq)NaCl(aq)+H2O(l)+CO2(g) Calculate the volume (in liters) of CO2 generated from 0.315g of NaHCO3 and excess gastric juice at 1.00atm and 37.00C. L
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


