Question: Enter your answer in the provided box. Calculate the pH of a buffer solution in which the acetic acid concentration is 6.3101M and the sodium
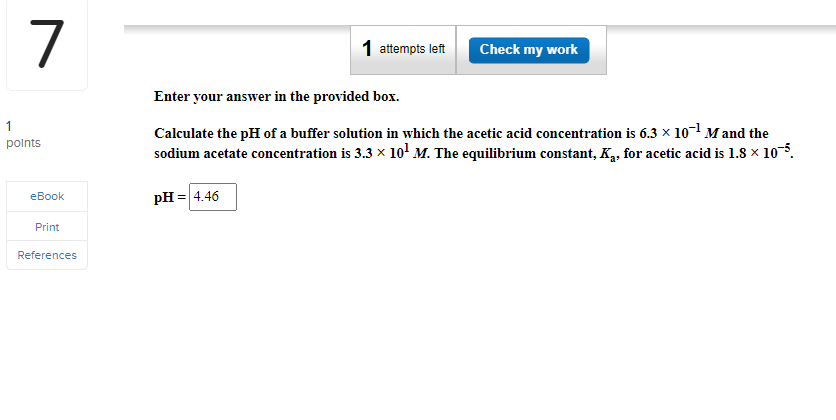
Enter your answer in the provided box. Calculate the pH of a buffer solution in which the acetic acid concentration is 6.3101M and the sodium acetate concentration is 3.3101M. The equilibrium constant, Ka, for acetic acid is 1.8105
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


