Question: Enter your answer in the provided box. A buffer solution is prepared in such a way that the concentration of propanoic acid is 8.28101M and
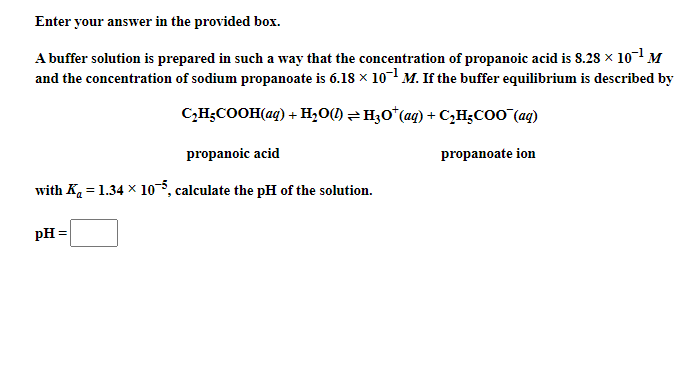
Enter your answer in the provided box. A buffer solution is prepared in such a way that the concentration of propanoic acid is 8.28101M and the concentration of sodium propanoate is 6.18101M. If the buffer equilibrium is described by C2H5COOH(aq)+H2O(l)H3O+(aq)+C2H5COO(aq) propanoic acid propanoate ion with Ka=1.34105, calculate the pH of the solution
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


