Question: Enter your answer in the provided box. From the data below, calculate the total heat (in J) needed to convert 30.00g of ice at 2.50C
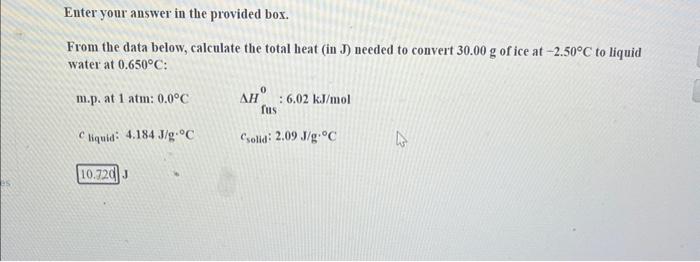
Enter your answer in the provided box. From the data below, calculate the total heat (in J) needed to convert 30.00g of ice at 2.50C to liquid water at 0.650C : m.p. at 1atm:0.0CHfus0:6.02kJ/mol cliquid:4.184J/gCcsolid:2.09J/gC
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


