Question: Please I need help answering these questions Enter your answer in the provided box. From the data below, calculate the total heat in J) needed



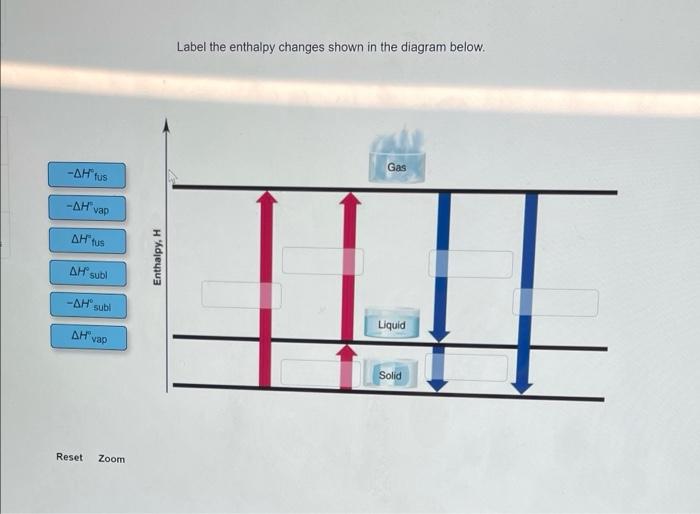

Enter your answer in the provided box. From the data below, calculate the total heat in J) needed to convert 0.160 mol of gaseous ethanol at 300.0C and 1 atm to liquid ethanol at 25.0C and 1 atm: b.p. at 1 atm: 78.5C An : 40.5 kJ/mol vap 1.43 J/g C Cliquid: 2.45 J/g C J es Enter your answer in the provided box. 0 a A liquid has a n of 33.6 kJ/mol and a boiling point of 133C at 1 atm. What is its vapor pressure vap at 113C atm ook int ences Enter your answer in the provided box. What is the hof a liquid that has a vapor pressure of 611 torr at 79.2C and a boiling point of vap 99.3C at 1 atm? J/mol Label the enthalpy changes shown in the diagram below. Gas -AHtus - Vap 1 AH tus Enthalpy, H AH sul -AH sub Liquid Solid Reset Zoom Be sure to answer all parts. 0.37 points In the following pairs, which substance has the lower boiling point? O Lici Print (b) O PH3 O NH References ( (c) o
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


