Question: Enter your answer in the provided box. How many total moles of ions are released when the following sample dissolves completely in water? 0.28mol of
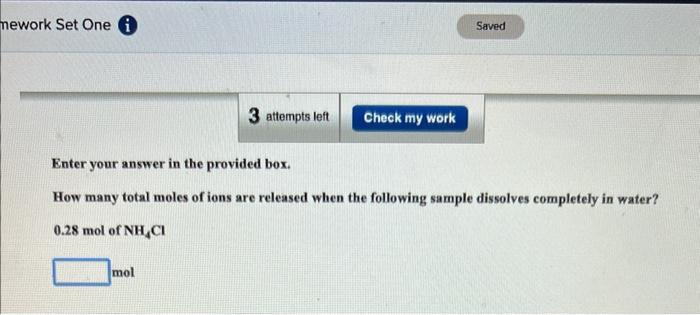
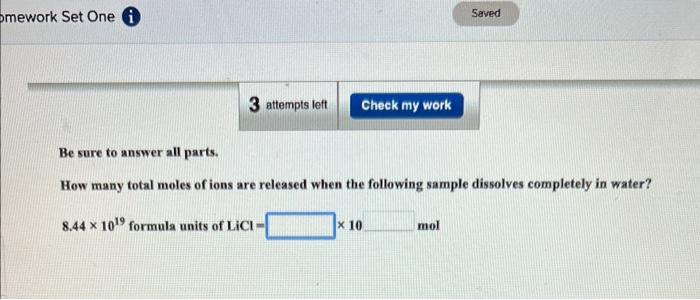
Enter your answer in the provided box. How many total moles of ions are released when the following sample dissolves completely in water? 0.28mol of NH4Cl mol Be sure to answer all parts. How many total moles of ions are released when the following sample dissolves completely in water? 8.441019formulaunitsofLiCl=|10mol
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


