Question: Enter your answer in the provided box. In a bromine-producing plant, how many liters of gaseous elemental bromine at 270. C and 0.989 atm are
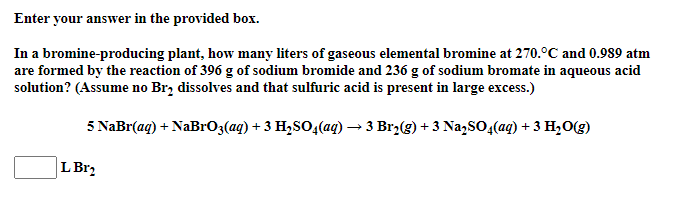
Enter your answer in the provided box. In a bromine-producing plant, how many liters of gaseous elemental bromine at 270. C and 0.989 atm are formed by the reaction of 396 g of sodium bromide and 236 g of sodium bromate in aqueous acid solution? (Assume no Br, dissolves and that sulfuric acid is present in large excess.) 5 NaBr(aq) + NaBrO3(aq) + 3 H2SO4(aq) 3 Br2(g) + 3 Na2SO4(aq) + 3 H20() + L Br2
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


