Question: Enter your answer in the provided box. The equilibrium constant Kc for the equation 2H2(g)+CO(g)CH3OH(g) is 25 at a certain temperature. If there are 6.71102
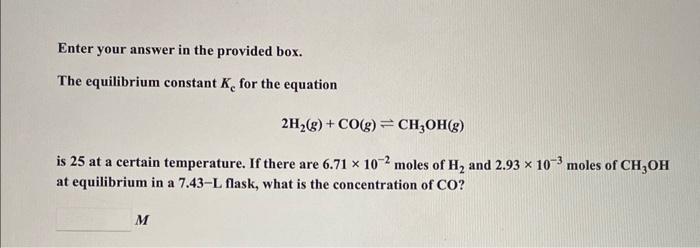
Enter your answer in the provided box. The equilibrium constant Kc for the equation 2H2(g)+CO(g)CH3OH(g) is 25 at a certain temperature. If there are 6.71102 moles of H2 and 2.93103 moles of CH3OH at equilibrium in a 7.43-L flask, what is the concentration of CO
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


