Question: Enter your answer in the provided box. The equilibrium constant for the equation 2H2(g) + CO() =CH2OH() is 27 at a certain temperature. If there
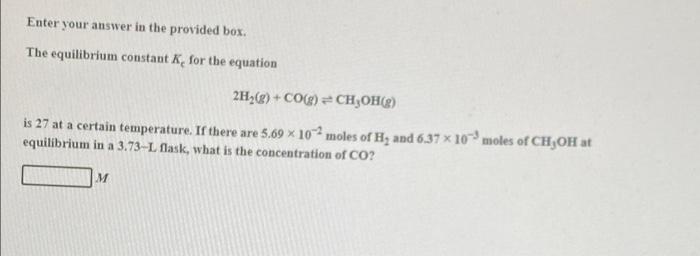
Enter your answer in the provided box. The equilibrium constant for the equation 2H2(g) + CO() =CH2OH() is 27 at a certain temperature. If there are 5.69 102 moles of Hy and 6,37 x 10 moles of CH,OH at equilibrium in a 3.73-L flask, what is the concentration of CO? M
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


