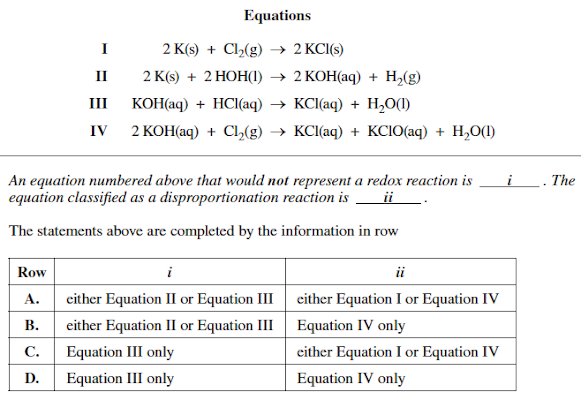
Question: Equations I 2 K ( s ) + C l 2 ( g ) 2 K C l ( s ) I I 2 K
Equations
I
HOHKOH
III KOH
KOHKClO
An equation numbered above that would not represent a redox reaction is The equation classified as a disproportionation reaction is ii
The statements above are completed by the information in row
tableRow
Step by Step Solution
There are 3 Steps involved in it
1 Expert Approved Answer
Step: 1 Unlock


Question Has Been Solved by an Expert!
Get step-by-step solutions from verified subject matter experts
Step: 2 Unlock
Step: 3 Unlock


