Question: Equilibrium Concentrations - A + B = 2C At a particular temperature, K = 1.00x102 for the reaction: H2(g) + F2(g) + 2HF(9) In an
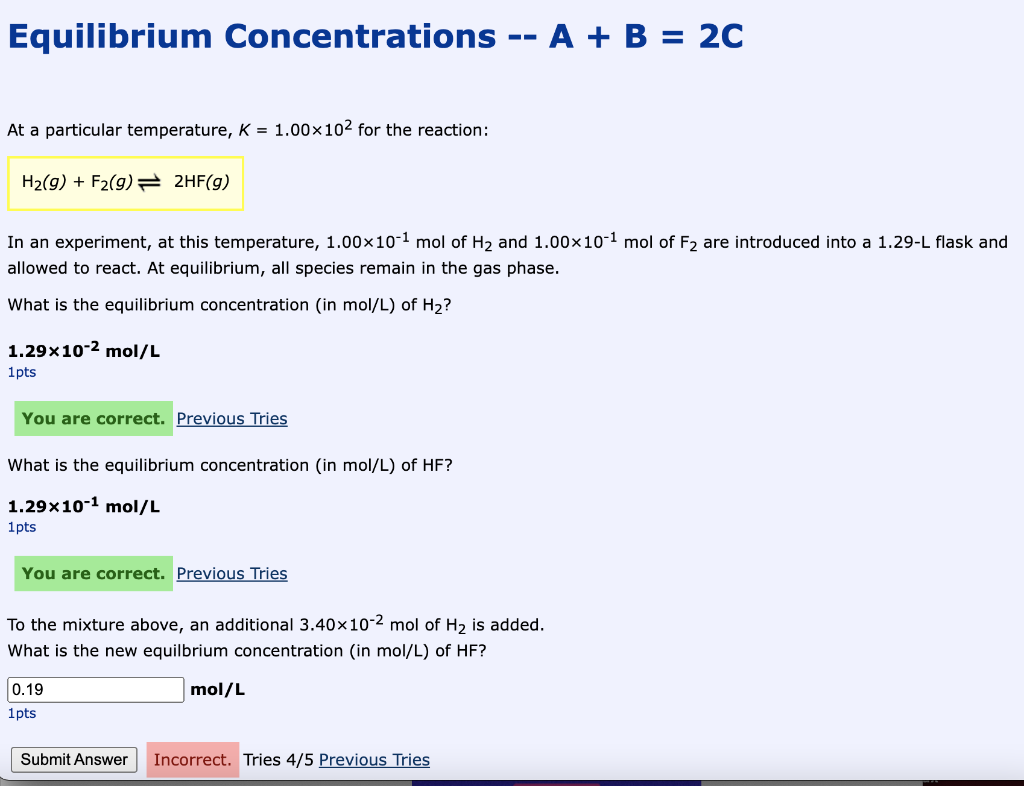
Equilibrium Concentrations - A + B = 2C At a particular temperature, K = 1.00x102 for the reaction: H2(g) + F2(g) + 2HF(9) In an experiment, at this temperature, 1.0010-1 mol of H2 and 1.00x10-1 mol of F2 are introduced into a 1.29-L flask and allowed to react. At equilibrium, all species remain in the gas phase. What is the equilibrium concentration (in mol/L) of H2? 1.29x10-2 mol/L 1 pts You are correct. Previous Tries What is the equilibrium concentration in mol/L) of HF? 1.29x10-1 mol/L 1pts You are correct. Previous Tries To the mixture above, an additional 3.40x10-2 mol of H2 is added. What is the new equilbrium concentration (in mol/L) of HF? mol/L 0.19 1pts Submit Answer Incorrect. Tries 4/5 Previous Tries
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


