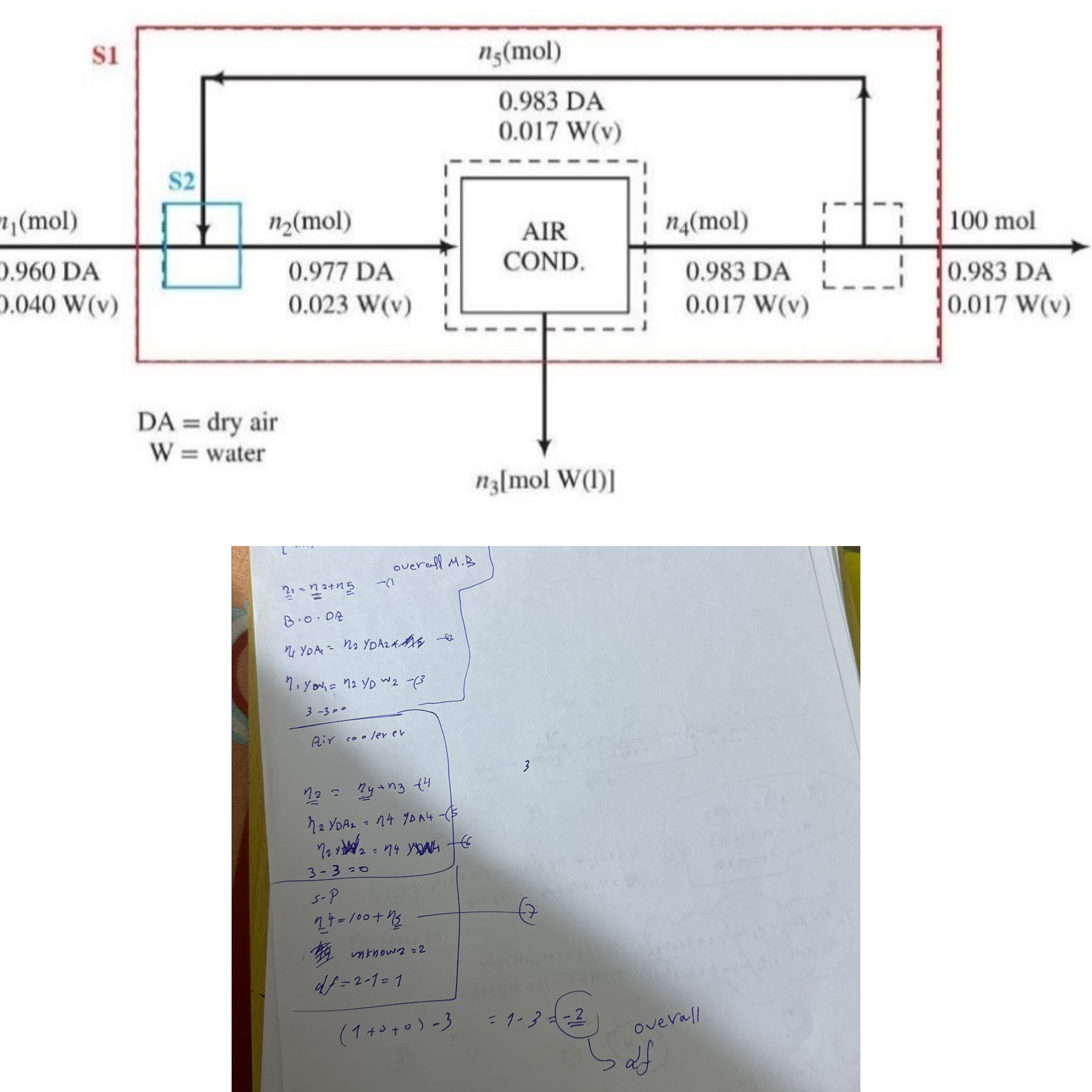
Question: esh air containing 4 . 0 0 mole % water vapor is to be cooled and dehumidified to a water content of 1 . 7
esh air containing mole water vapor is to be cooled and dehumidified to a water content of mole HO A stream of fresh air is combined with a recycle stream of previously dehumidified air and passed through the cooler. The blended stream entering the unit contains mole HO In the air conditioner, some of the water in the feed stream is condensed and removed as liquid. A fraction of the dehumidified air leaving the cooler is recycled and the remainder is delivered to a room. Write the equations that simulate above process. Perform the degree of freedom for the overall and sub processes List your answer in table Calculate the moles of fresh feed, moles of water condensed, and moles of dehumidified air recycled
I dont need tge solution i just need to know if i got the overalldf right
Can u recorrect,?
Step by Step Solution
There are 3 Steps involved in it
1 Expert Approved Answer
Step: 1 Unlock


Question Has Been Solved by an Expert!
Get step-by-step solutions from verified subject matter experts
Step: 2 Unlock
Step: 3 Unlock


