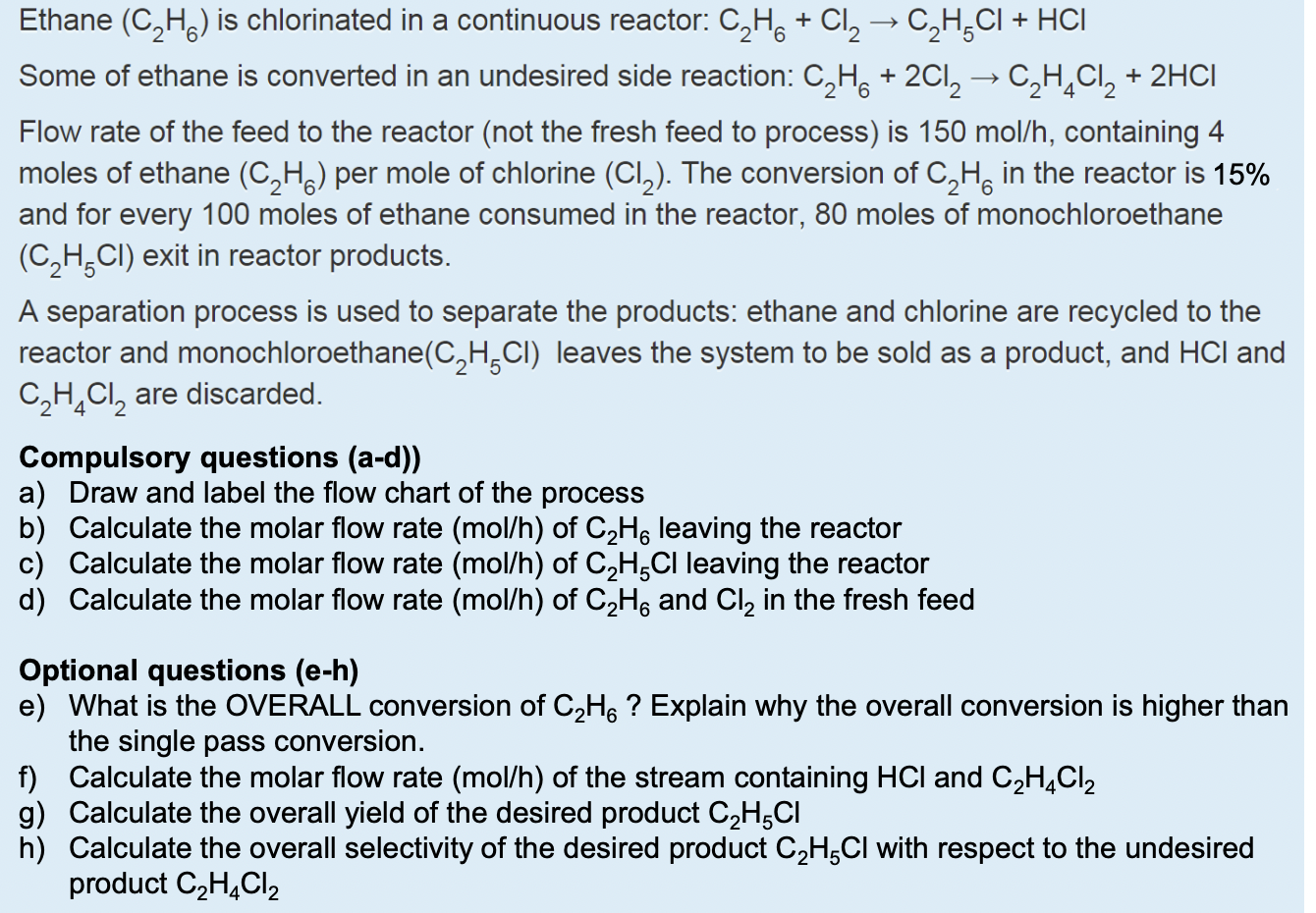
Question: Ethane ( C 2 H 6 ) is chlorinated in a continuous reactor: C 2 H 6 + C l 2 C 2 H 5
Ethane is chlorinated in a continuous reactor:
Some of ethane is converted in an undesired side reaction:
Flow rate of the feed to the reactor not the fresh feed to process is containing
moles of ethane per mole of chlorine The conversion of in the reactor is
and for every moles of ethane consumed in the reactor, moles of monochloroethane
exit in reactor products.
A separation process is used to separate the products: ethane and chlorine are recycled to the
reactor and monochloroethane leaves the system to be sold as a product, and and
are discarded.
Compulsory questions ad
a Draw and label the flow chart of the process
b Calculate the molar flow rate of leaving the reactor
c Calculate the molar flow rate of leaving the reactor
d Calculate the molar flow rate of and in the fresh feed
Optional questions eh
e What is the OVERALL conversion of Explain why the overall conversion is higher than
the single pass conversion.
f Calculate the molar flow rate of the stream containing and
g Calculate the overall yield of the desired product
h Calculate the overall selectivity of the desired product with respect to the undesired
product
Step by Step Solution
There are 3 Steps involved in it
1 Expert Approved Answer
Step: 1 Unlock


Question Has Been Solved by an Expert!
Get step-by-step solutions from verified subject matter experts
Step: 2 Unlock
Step: 3 Unlock


