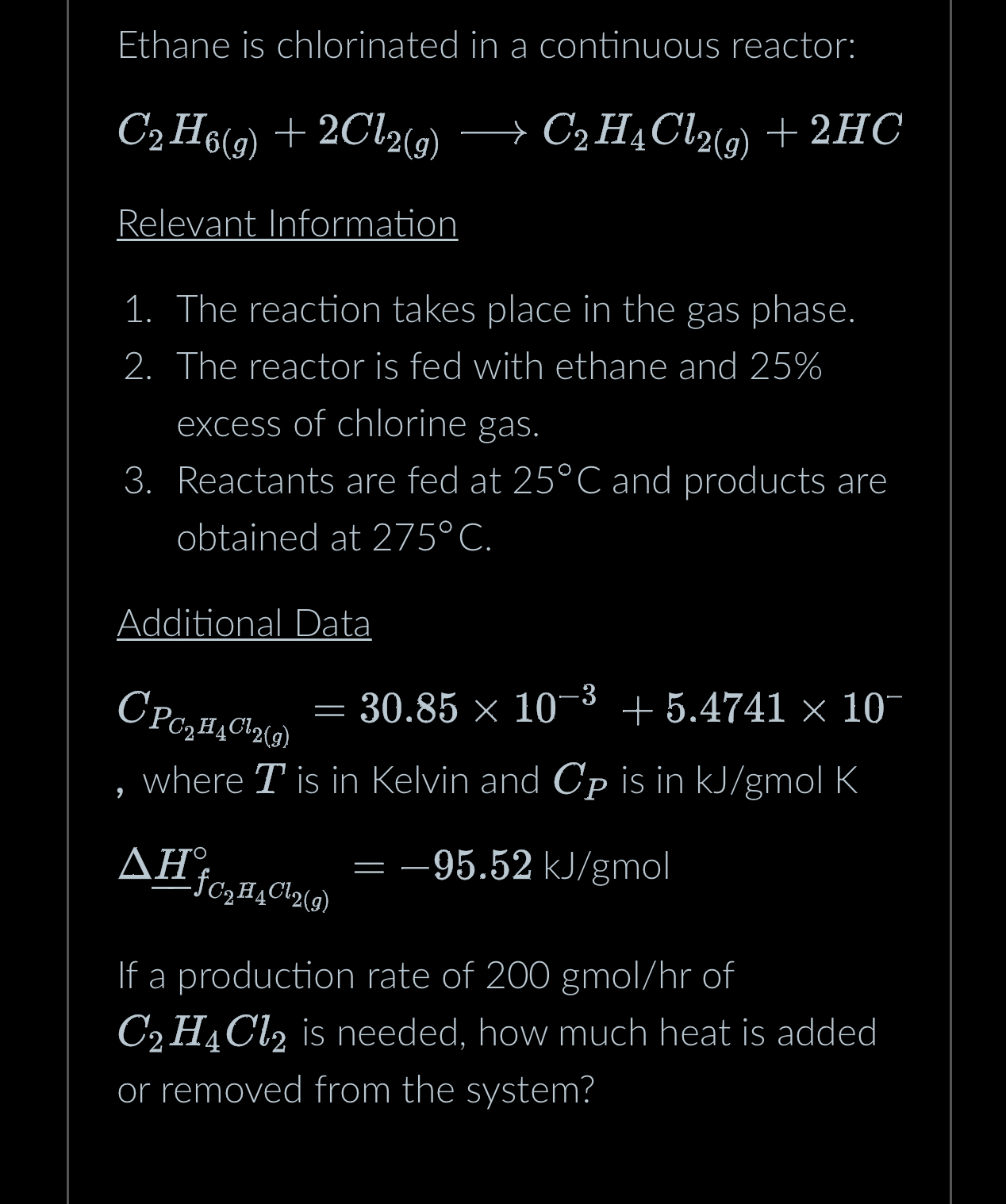
Question: Ethane is chlorinated in a continuous reactor: C 2 H 6 ( g ) + 2 C l 2 ( g ) l o n
Ethane is chlorinated in a continuous reactor:
Relevant Information
The reaction takes place in the gas phase.
The reactor is fed with ethane and excess of chlorine gas.
Reactants are fed at and products are obtained at
Additional Data
where is in Kelvin and is in molK
mol
If a production rate of gmo of is needed, how much heat is added or removed from the system?
Step by Step Solution
There are 3 Steps involved in it
1 Expert Approved Answer
Step: 1 Unlock


Question Has Been Solved by an Expert!
Get step-by-step solutions from verified subject matter experts
Step: 2 Unlock
Step: 3 Unlock


