Question: Example 1-2 How Large Is It? Consider the liquid phase cis - trans isomerization of 2-butene H H H CH CH CH; CH, H H
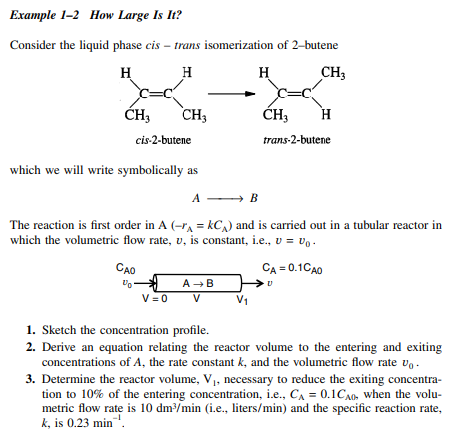
Example 1-2 How Large Is It? Consider the liquid phase cis - trans isomerization of 2-butene H H H CH CH CH; CH, H H cis-2-butene trans-2-butene which we will write symbolically as A - B The reaction is first order in A (r^ = kC) and is carried out in a tubular reactor i which the volumetric flow rate, v, is constant, i.e., V = Vo. CAO CA = 0.1CAO AB VEO V VO 1. Sketch the concentration profile. 2. Derive an equation relating the reactor volume to the entering and exiting concentrations of A, the rate constant k, and the volumetric flow rate vo. 3. Determine the reactor volume, V, necessary to reduce the exiting concentra- tion to 10% of the entering concentration, i.e., CA = 0.1Co when the volu- metric flow rate is 10 dm3/min (i.e., liters/min) and the specific reaction rate, k, is 0.23 min
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


