Question: > U Care . mo Chad Terit POUDA Example 1-2 How Large Is the Reactor Volume? Let's consider the liquid phase cis-trans isomerization of 2-butene
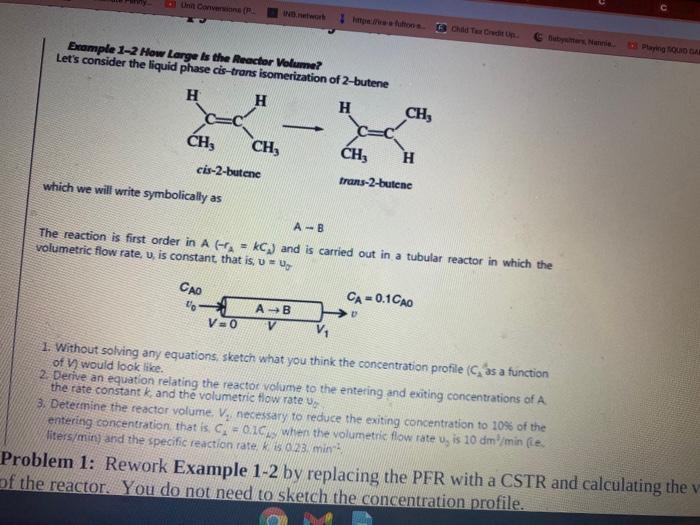
> U Care . mo Chad Terit POUDA Example 1-2 How Large Is the Reactor Volume? Let's consider the liquid phase cis-trans isomerization of 2-butene H H H CH, . CH, CH, CH, H cis-2-butene trans-2-bulenc which we will write symbolically as A-B The reaction is first order in A (A - KC) and is carried out in a tubular reactor in which the volumetric flow rate, v, is constant that is, muy CA = 0.1CAO AB V=0 CAD 1. Without solving any equations, sketch what you think the concentration profile (C as a function of would look like. 2. Derive an equation relating the reactor volume to the entering and exiting concentrations of A the rate constant and the volumetric flow rate 3. Determine the reactor volume. Vy necessary to reduce the exiting concentration to 10% of the entering concentration that is C. - 01C when the volumetric flow rate u, is 10 dm/min (e. liters/min) and the specific reaction rate kis 023 min Problem 1: Rework Example 1-2 by replacing the PFR with a CSTR and calculating the v of the reactor. You do not need to sketch the concentration profile
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


