Question: Example 2.7 Air at 1 bar and 298.15K is compressed to 3 bar and 298.15K in a closed-system mechanically reversible process first by heating at
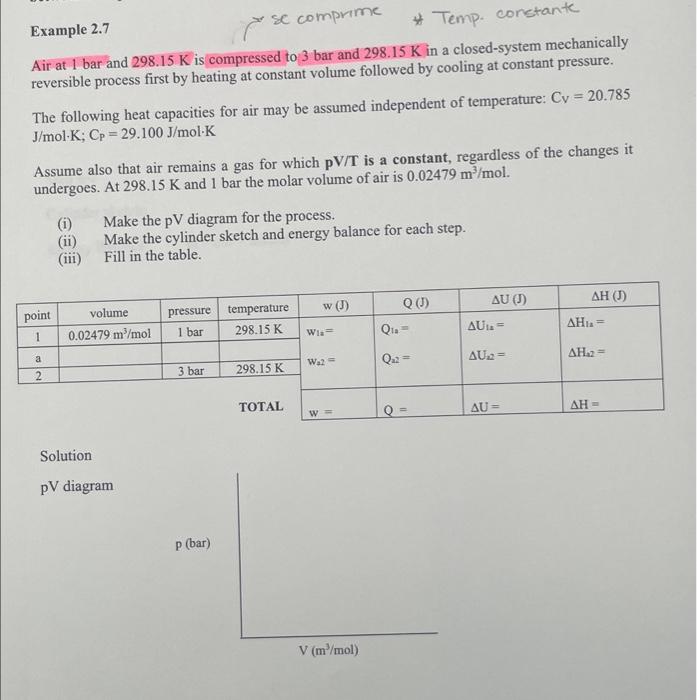

Example 2.7 Air at 1 bar and 298.15K is compressed to 3 bar and 298.15K in a closed-system mechanically reversible process first by heating at constant volume followed by cooling at constant pressure. The following heat capacities for air may be assumed independent of temperature: Cv=20.785 J/molK;CP=29.100J/molK Assume also that air remains a gas for which pV/T is a constant, regardless of the changes it undergoes. At 298.15K and 1 bar the molar volume of air is 0.02479m3/mol. (i) Make the pV diagram for the process. (ii) Make the cylinder sketch and energy balance for each step. (iii) Fill in the table. Solution pV diagram Step 1-a Cylinder sketch: Energy Balance: inout=accum Step a-2 Cylinder sketch: Energy Balance: in - out = accum
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


