Question: Example 5.2 Natural gas often will contain CO2 and can be purified by gas permeation through a variety of polymeric membranes. A natural gas well
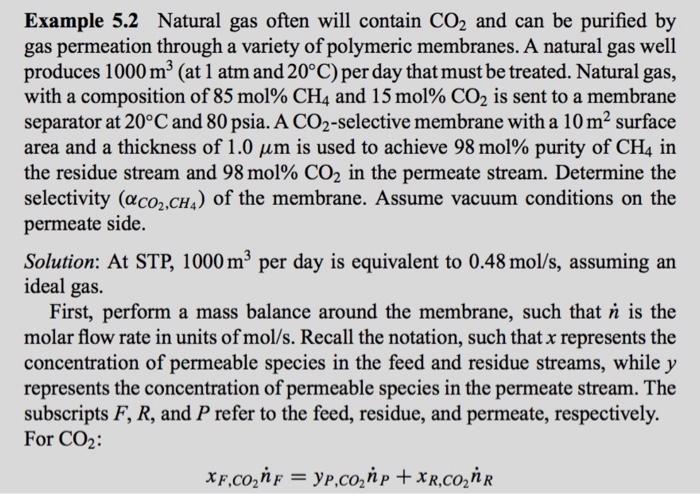
Example 5.2 Natural gas often will contain CO2 and can be purified by gas permeation through a variety of polymeric membranes. A natural gas well produces 1000m3 (at 1atm and 20C ) per day that must be treated. Natural gas, separator at 20C and 80psia.ACO2-selective membrane with a 10m2 surface area and a thickness of 1.0m is used to achieve 98mol% purity of CH4 in the residue stream and 98mol%CO2 in the permeate stream. Determine the selectivity (CO2,CH4) of the membrane. Assume vacuum conditions on the permeate side. Solution: At STP, 1000m3 per day is equivalent to 0.48mol/s, assuming an ideal gas. First, perform a mass balance around the membrane, such that n is the molar flow rate in units of mol/s. Recall the notation, such that x represents the concentration of permeable species in the feed and residue streams, while y represents the concentration of permeable species in the permeate stream. The subscripts F,R, and P refer to the feed, residue, and permeate, respectively. For CO2 : xF,CO2nF=yP,CO2nP+xR,CO2nR
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


