Question: Example 6.15: Gas-Phase Reaction in a Packed Bed Reactor 19 The irreversible gas-phase catalytic reaction A+BC+D is to be carried out in a packed bed
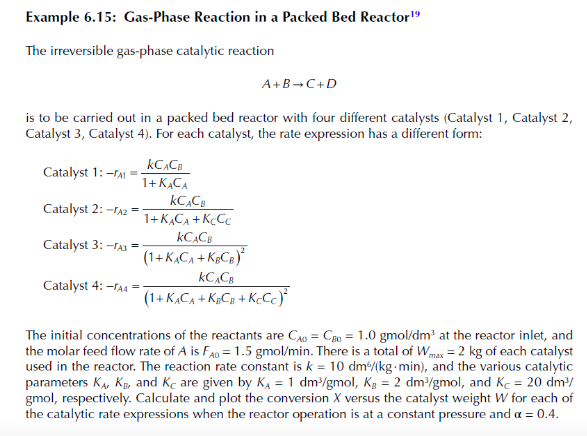
Example 6.15: Gas-Phase Reaction in a Packed Bed Reactor 19 The irreversible gas-phase catalytic reaction A+BC+D is to be carried out in a packed bed reactor with four different catalysts (Catalyst 1, Catalyst 2 , Catalyst 3, Catalyst 4). For each catalyst, the rate expression has a different form: Catalyst1:rA1=1+KACAkCACBCatalyst2:rA2=1+KACA+KCCCkCACBCatalyst3:rA3=(1+KACA+KBCB)2kCACBCatalyst4:rA4=(1+KACA+KBCB+KCCC)2kCACB The initial concentrations of the reactants are CAO=CBO=1.0gmol/dm3 at the reactor inlet, and the molar feed flow rate of A is FA0=1.5gmol/min. There is a total of Wmax=2kg of each catalyst used in the reactor. The reaction rate constant is k=10dm/(kgmin), and the various catalytic parameters KA,KE and KC are given by KA=1dm3/gmolKB=2dm3/gmol, and KC=20dm3/ gmol, respectively. Calculate and plot the conversion X versus the catalyst weight W for each of the catalytic rate expressions when the reactor operation is at a constant pressure and =0.4
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


